Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies
- PMID: 34253574
- PMCID: PMC8862014
- DOI: 10.1136/gutjnl-2020-323392
Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies
Abstract
Objective: In this study, we determined whether Helicobacter pylori (H. pylori) infection dampens the efficacy of cancer immunotherapies.
Design: Using mouse models, we evaluated whether immune checkpoint inhibitors or vaccine-based immunotherapies are effective in reducing tumour volumes of H. pylori-infected mice. In humans, we evaluated the correlation between H. pylori seropositivity and the efficacy of the programmed cell death protein 1 (PD-1) blockade therapy in patients with non-small-cell lung cancer (NSCLC).
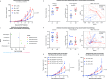
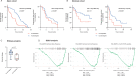
Results: In mice engrafted with MC38 colon adenocarcinoma or B16-OVA melanoma cells, the tumour volumes of non-infected mice undergoing anticytotoxic T-lymphocyte-associated protein 4 and/or programmed death ligand 1 or anti-cancer vaccine treatments were significantly smaller than those of infected mice. We observed a decreased number and activation status of tumour-specific CD8+ T cells in the tumours of infected mice treated with cancer immunotherapies independent of the gut microbiome composition. Additionally, by performing an in vitro co-culture assay, we observed that dendritic cells of infected mice promote lower tumour-specific CD8+ T cell proliferation. We performed retrospective human clinical studies in two independent cohorts. In the Dijon cohort, H. pylori seropositivity was found to be associated with a decreased NSCLC patient survival on anti-PD-1 therapy. The survival median for H. pylori seropositive patients was 6.7 months compared with 15.4 months for seronegative patients (p=0.001). Additionally, in the Montreal cohort, H. pylori seropositivity was found to be associated with an apparent decrease of NSCLC patient progression-free survival on anti-PD-1 therapy.
Conclusion: Our study unveils for the first time that the stomach microbiota affects the response to cancer immunotherapies and that H. pylori serology would be a powerful tool to personalize cancer immunotherapy treatment.
Keywords: Helicobacter pylori; cancer; cancer immunobiology; immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FG received honoraria for oral communication from Lilly, Sanofi, Amgen, and is an advisory board of Merck Serono, Amgen and Sanofi. The remaining authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
