Overcoming Intrinsic Resistance of Cancer Cells to CAR T-Cell Killing
- PMID: 34253582
- PMCID: PMC11260069
- DOI: 10.1158/1078-0432.CCR-21-1559
Overcoming Intrinsic Resistance of Cancer Cells to CAR T-Cell Killing
Abstract
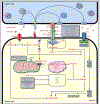
In the past few years, chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising treatment for cancers that failed standard treatments. Such therapies have already been approved in several blood cancers, such as B-cell leukemia and lymphoma. Despite this progress, a significant proportion of patients experience primary or secondary resistance to CAR T-cell therapy. Here, we review the mechanisms by which CAR T cells eliminate their target and how cancer cells may be insensitive to such killing (here referred to as intrinsic resistance). Recent studies suggest that the activation of apoptosis through death receptor signaling is responsible for a major part of CAR T-cell cytotoxicity in vivo Indeed, cancer cells harboring aberrant apoptotic machinery may be insensitive to CAR T-cell killing. This intrinsic resistance of cancer cells to CAR T-cell killing could be responsible for a significant portion of treatment failure. Finally, we discuss strategies that may be envisioned to overcome such resistance to enhance CAR T-cell efficacy.
©2021 American Association for Cancer Research.
Figures


References
-
- Lamers CHJ, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo–engineered T cells. Blood 2011; 117:72–82. - PubMed
-
- Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev 2014; 257:14–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

