Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo
- PMID: 34253590
- PMCID: PMC9398105
- DOI: 10.1158/1535-7163.MCT-21-0035
Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo
Abstract
B-cell maturation antigen (BCMA) is an attractive therapeutic target highly expressed on differentiated plasma cells in multiple myeloma and other B-cell malignancies. GSK2857916 (belantamab mafodotin, BLENREP) is a BCMA-targeting antibody-drug conjugate approved for the treatment of relapsed/refractory multiple myeloma. We report that GSK2857916 induces immunogenic cell death in BCMA-expressing cancer cells and promotes dendritic cell activation in vitro and in vivo GSK2857916 treatment enhances intratumor immune cell infiltration and activation, delays tumor growth, and promotes durable complete regressions in immune-competent mice bearing EL4 lymphoma tumors expressing human BCMA (EL4-hBCMA). Responding mice are immune to rechallenge with EL4 parental and EL4-hBCMA cells, suggesting engagement of an adaptive immune response, immunologic memory, and tumor antigen spreading, which are abrogated upon depletion of endogenous CD8+ T cells. Combinations with OX40/OX86, an immune agonist antibody, significantly enhance antitumor activity and increase durable complete responses, providing a strong rationale for clinical evaluation of GSK2857916 combinations with immunotherapies targeting adaptive immune responses, including T-cell-directed checkpoint modulators.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures
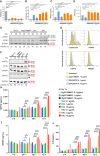
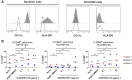
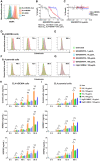
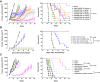


References
-
- Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235–47. - PubMed
-
- Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol 1998;161:6510–7. - PubMed
-
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

