The 2021 update of the EPA's adverse outcome pathway database
- PMID: 34253739
- PMCID: PMC8275694
- DOI: 10.1038/s41597-021-00962-3
The 2021 update of the EPA's adverse outcome pathway database
Abstract
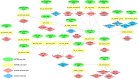
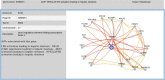
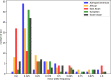
The EPA developed the Adverse Outcome Pathway Database (AOP-DB) to better characterize adverse outcomes of toxicological interest that are relevant to human health and the environment. Here we present the most recent version of the EPA Adverse Outcome Pathway Database (AOP-DB), version 2. AOP-DB v.2 introduces several substantial updates, which include automated data pulls from the AOP-Wiki 2.0, the integration of tissue-gene network data, and human AOP-gene data by population, semantic mapping and SPARQL endpoint creation, in addition to the presentation of the first publicly available AOP-DB web user interface. Potential users of the data may investigate specific molecular targets of an AOP, the relation of those gene/protein targets to other AOPs, cross-species, pathway, or disease-AOP relationships, or frequencies of AOP-related functional variants in particular populations, for example. Version updates described herein help inform new testable hypotheses about the etiology and mechanisms underlying adverse outcomes of environmental and toxicological concern.
© 2021. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures
Dataset use reported in
- doi: 10.1007/s00335-018-9738-7
References
-
- US Public Law 114–182. Frank R. Lautenberg Chemical Safety for the 21st Century Act (ed 114th Congress, 2016).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

