Excess membrane binding of monomeric alpha-, beta- and gamma-synuclein is invariably associated with inclusion formation and toxicity
- PMID: 34254125
- PMCID: PMC8600006
- DOI: 10.1093/hmg/ddab188
Excess membrane binding of monomeric alpha-, beta- and gamma-synuclein is invariably associated with inclusion formation and toxicity
Abstract
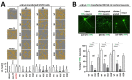
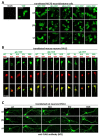
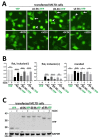
α-Synuclein (αS) has been well-documented to play a role in human synucleinopathies such as Parkinson's disease (PD) and dementia with Lewy bodies (DLB). First, the lesions found in PD/DLB brains-Lewy bodies and Lewy neurites-are rich in aggregated αS. Second, genetic evidence links missense mutations and increased αS expression to familial forms of PD/DLB. Third, toxicity and cellular stress can be caused by αS under certain experimental conditions. In contrast, the homologs β-synuclein (βS) and γ-synuclein (γS) are not typically found in Lewy bodies/neurites, have not been clearly linked to brain diseases and have been largely non-toxic in experimental settings. In αS, the so-called non-amyloid-β component of plaques (NAC) domain, constituting amino acids 61-95, has been identified to be critical for aggregation in vitro. This domain is partially absent in βS and only incompletely conserved in γS, which could explain why both homologs do not cause disease. However, αS in vitro aggregation and cellular toxicity have not been firmly linked experimentally, and it has been proposed that excess αS membrane binding is sufficient to induce neurotoxicity. Indeed, recent characterizations of Lewy bodies have highlighted the accumulation of lipids and membranous organelles, raising the possibility that βS and γS could also become neurotoxic if they were more prone to membrane/lipid binding. Here, we increased βS and γS membrane affinity by strategic point mutations and demonstrate that these proteins behave like membrane-associated monomers, are cytotoxic and form round cytoplasmic inclusions that can be prevented by inhibiting stearoyl-CoA desaturase.
© The Author(s) 2021. Published by Oxford University Press.
Figures

References
-
- Spillantini, M.G., Schmidt, M.L., Lee, V.M., Trojanowski, J.Q., Jakes, R. and Goedert, M. (1997) Alpha-synuclein in Lewy bodies. Nature, 388, 839–840. - PubMed
-
- Lavedan, C., Buchholtz, S., Auburger, G., Albin, R.L., Athanassiadou, A., Blancato, J., Burguera, J.A., Ferrell, R.E., Kostic, V., Leroy, E. et al. (1998) Absence of mutation in the beta- and gamma-synuclein genes in familial autosomal dominant Parkinson’s disease. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes, 5, 401–402. - PubMed
-
- Lincoln, S., Crook, R., Chartier-Harlin, M.C., Gwinn-Hardy, K., Baker, M., Mouroux, V., Richard, F., Becquet, E., Amouyel, P., Destée, A., Hardy, J., Farrera, M. (1999) No pathogenic mutations in the beta-synuclein gene in Parkinson’s disease. Neurosci. Lett., 269, 107–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

