Lung transplantation for acute respiratory distress syndrome: A multicenter experience
- PMID: 34254423
- PMCID: PMC8441742
- DOI: 10.1111/ajt.16759
Lung transplantation for acute respiratory distress syndrome: A multicenter experience
Abstract
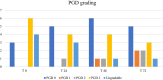
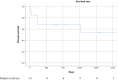
Acute respiratory distress syndrome (ARDS) is a rapidly progressive lung disease with a high mortality rate. Although lung transplantation (LTx) is a well-established treatment for a variety of chronic pulmonary diseases, LTx for acute lung failure (due to ARDS) remains controversial. We reviewed posttransplant outcome of ARDS patients from three high-volume European transplant centers. Demographics and clinical data were collected and analyzed. Viral infection was the main reason for ARDS (n = 7/13, 53.8%). All patients were admitted to ICU and required mechanical ventilation, 11/13 were supported with ECMO at the time of listing. They were granted a median LAS of 76 (IQR 50-85) and waited for a median of 3 days (IQR 1.5-14). Postoperatively, median length of mechanical ventilation was 33 days (IQR 17-52.5), median length of ICU and hospital stay were 39 days (IQR 19.5-58.5) and 54 days (IQR 43.5-127). Prolongation of peripheral postoperative ECMO was required in 7/13 (53.8%) patients with a median duration of 2 days (IQR 2-7). 30-day mortality was 7.7%, 1 and 5-year survival rates were calculated as 71.6% and 54.2%, respectively. Given the lack of alternative treatment options, the herein presented results support the concept of offering live-saving LTx to carefully selected ARDS patients.
Keywords: clinical research/practice; health services and outcomes research; lung (allograft) function/dysfunction; lung failure/injury; lung transplantation/pulmonology.
© 2021 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
References
-
- Bernard GR, Artigas A, Brigham KL, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3):818‐824. - PubMed
-
- Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965‐1975. - PubMed
-
- Brodie D, Bacchetta M. Extracorporeal Membrane Oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905‐1914. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334‐1349. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

