Treatment Effect Measures for Culture Conversion Endpoints in Phase IIb Tuberculosis Treatment Trials
- PMID: 34254635
- PMCID: PMC8664460
- DOI: 10.1093/cid/ciab576
Treatment Effect Measures for Culture Conversion Endpoints in Phase IIb Tuberculosis Treatment Trials
Abstract
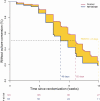
Phase IIb trials of tuberculosis therapy rely on early biomarkers of treatment effect. Despite limited predictive ability for clinical outcomes, culture conversion, the event in which an individual previously culture positive for Mycobacterium tuberculosis yields a negative culture after initiating treatment, is a commonly used endpoint. Lack of consensus on how to define the outcome and corresponding measure of treatment effect complicates interpretation and limits between-trial comparisons. We review common analytic approaches to measuring treatment effect and introduce difference in restricted mean survival times as an alternative to identify faster times to culture conversion and express magnitude of effect on the time scale. Findings from the PanACEA MAMS-TB trial are reanalyzed as an illustrative example. In a systematic review we demonstrate variability in analytic approaches, sampling strategies, and outcome definitions in phase IIb tuberculosis trials. Harmonization would allow for larger meta-analyses and may help expedite advancement of new tuberculosis therapeutics.
Keywords: clinical trials; phase II; randomized controlled trials; survival analysis; tuberculosis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Global tuberculosis report. 2020. Geneva, Switzerland: World Health Organization; 2020.
-
- Wallis RS, Doherty TM, Onyebujoh P, et al. . Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 2009; 9:162–72. - PubMed
-
- Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 1993; 147:1062–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

