Microcircuits for spatial coding in the medial entorhinal cortex
- PMID: 34254836
- PMCID: PMC8759973
- DOI: 10.1152/physrev.00042.2020
Microcircuits for spatial coding in the medial entorhinal cortex
Abstract
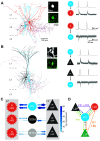
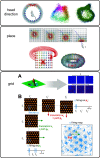
The hippocampal formation is critically involved in learning and memory and contains a large proportion of neurons encoding aspects of the organism's spatial surroundings. In the medial entorhinal cortex (MEC), this includes grid cells with their distinctive hexagonal firing fields as well as a host of other functionally defined cell types including head direction cells, speed cells, border cells, and object-vector cells. Such spatial coding emerges from the processing of external inputs by local microcircuits. However, it remains unclear exactly how local microcircuits and their dynamics within the MEC contribute to spatial discharge patterns. In this review we focus on recent investigations of intrinsic MEC connectivity, which have started to describe and quantify both excitatory and inhibitory wiring in the superficial layers of the MEC. Although the picture is far from complete, it appears that these layers contain robust recurrent connectivity that could sustain the attractor dynamics posited to underlie grid pattern formation. These findings pave the way to a deeper understanding of the mechanisms underlying spatial navigation and memory.
Keywords: connectivity; entorhinal cortex; grid cells; microcircuits; navigation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

