Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis
- PMID: 34254978
- PMCID: PMC8278308
- DOI: 10.1001/jamainternmed.2021.4325
Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis
Abstract
Importance: Two mRNA-based vaccines against coronavirus disease 2019 (COVID-19) were found to be highly efficacious in phase 3 clinical trials in the US. However, patients with chronic illnesses, including cirrhosis, were excluded from clinical trials. Patients with cirrhosis have immune dysregulation that is associated with vaccine hyporesponsiveness.
Objective: To study the association of receipt of the Pfizer BNT162b2 mRNA or the Moderna mRNA-1273 vaccines in patients with cirrhosis compared with a propensity-matched control group of patients at similar risk of infection and severe disease from COVID-19.
Design, setting, and participants: We performed a retrospective cohort study of patients with cirrhosis who received at least 1 dose of a COVID-19 mRNA vaccine at the Veterans Health Administration. Patients who received at least 1 dose of the vaccine (n = 20 037) were propensity matched with 20 037 controls to assess the associations of vaccination with new COVID-19 infection and COVID-19 hospitalization and death.
Exposures: Receipt of at least 1 dose of the BNT162b2 mRNA or the mRNA-1273 vaccines between December 18, 2020, and March 17, 2021.
Main outcomes and measures: COVID-19 infection as documented by a positive result for COVID-19 by polymerase chain reaction, hospitalization, and death due to COVID-19 infection.
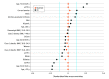
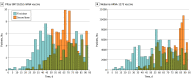
Results: The median (interquartile range) age of the vaccinated individuals in the study cohort was 69.1 (8.4) years and 19 465 (97.2%) of the participants in each of the vaccinated and unvaccinated groups were male, consistent with a US veteran population. The mRNA-1273 vaccine was administered in 10 236 (51%) and the BNT162b2 mRNA in 9801 (49%) patients. Approximately 99.7% of patients who received the first dose of either vaccine with a follow-up of 42 days or more received a second dose. The number of COVID-19 infections in the vaccine recipients was similar to the control group in days 0 to 7, 7 to 14, 14 to 21, and 21 to 28 after the first dose. After 28 days, receipt of 1 dose of an mRNA vaccine was associated with a 64.8% reduction in COVID-19 infections and 100% protection against hospitalization or death due to COVID-19 infection. The association of reduced COVID-19 infections after the first dose was lower among patients with decompensated (50.3%) compared with compensated cirrhosis (66.8%). Receipt of a second dose was associated with a 78.6% reduction in COVID-19 infections and 100% reduction in COVID-19-related hospitalization or death after 7 days.
Conclusions and relevance: This cohort study of US veterans found that mRNA vaccine administration was associated with a delayed but modest reduction in COVID-19 infection but an excellent reduction in COVID-19-related hospitalization or death in patients with cirrhosis.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

