Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial
- PMID: 34255009
- PMCID: PMC8278266
- DOI: 10.1001/jama.2021.6986
Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial
Abstract
Importance: Platelet-rich plasma injections are used as a treatment for chronic midportion Achilles tendinopathy, but evidence for this treatment is limited.
Objective: In adults with midportion Achilles tendinopathy, to assess the effects of a single platelet-rich plasma injection, compared with sham injection, on the outcome of the Victorian Institute of Sport Assessment-Achilles (VISA-A) score (a single composite measure of Achilles tendinopathy severity).
Design, setting, and participants: A participant-blinded, multicenter randomized clinical trial that included 240 people from 24 sites assigned to either a platelet-rich plasma injection or a sham injection between April 2016 and February 2020. Final follow-up was July 2020. Participants were older than 18 years with midportion Achilles tendon pain for more than 3 months as confirmed by ultrasound, magnetic resonance imaging, or both.
Interventions: A single intratendinous platelet-rich plasma injection (n = 121) or a single sham injection (insertion of a subcutaneous dry needle not entering the tendon) (n = 119).
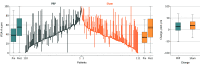
Main outcomes and measures: The primary outcome was the VISA-A score, measured 6 months after treatment allocation. The VISA-A score contains 8 questions that cover 3 domains of pain, function, and activity, analyzed as a composite score (range, 0 [worst symptoms] to 100 [no symptoms]; minimal clinically important difference in score, 12 points). The primary analysis was adjusted for laterality, age, sex, and baseline VISA-A score.
Results: Among 240 patients assigned to a platelet-rich plasma or sham injection (mean age, 52 years; 138 [58%] women), 221 (92%) completed the trial. At 6-month follow-up, mean VISA-A score values in the plasma-rich plasma group vs the sham injection group were 54.4 vs 53.4 (adjusted mean difference, -2.7 [95% CI, -8.8 to 3.3]). The most common adverse events compared between patients in the platelet-rich plasma group vs the sham group were injection site discomfort (97 vs 73 patients), swelling (56 vs 52 patients) and bruising (48 vs 49 patients).
Conclusions and relevance: Among patients with chronic midportion Achilles tendinopathy, treatment with a single injection of intratendinous platelet-rich plasma, compared with insertion of a subcutaneous dry needle, did not reduce Achilles tendon dysfunction at 6 months. These findings do not support the use of this treatment for chronic midportion Achilles tendinopathy.
Trial registration: isrctn.org Identifier: ISRCTN13254422.
Conflict of interest statement
Figures

Comment in
-
Platelet-Rich Plasma Injection vs Sham Injection and Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy.JAMA. 2021 Nov 16;326(19):1974. doi: 10.1001/jama.2021.16055. JAMA. 2021. PMID: 34783845 No abstract available.
-
Platelet-Rich Plasma Injection vs Sham Injection and Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy.JAMA. 2021 Nov 16;326(19):1974-1975. doi: 10.1001/jama.2021.16052. JAMA. 2021. PMID: 34783846 No abstract available.
-
Platelet-Rich Plasma Injection vs Sham Injection and Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy.JAMA. 2021 Nov 16;326(19):1975. doi: 10.1001/jama.2021.16049. JAMA. 2021. PMID: 34783847 No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

