Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative
- PMID: 34255046
- PMCID: PMC8278272
- DOI: 10.1001/jamanetworkopen.2021.16901
Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative
Abstract
Importance: The National COVID Cohort Collaborative (N3C) is a centralized, harmonized, high-granularity electronic health record repository that is the largest, most representative COVID-19 cohort to date. This multicenter data set can support robust evidence-based development of predictive and diagnostic tools and inform clinical care and policy.
Objectives: To evaluate COVID-19 severity and risk factors over time and assess the use of machine learning to predict clinical severity.
Design, setting, and participants: In a retrospective cohort study of 1 926 526 US adults with SARS-CoV-2 infection (polymerase chain reaction >99% or antigen <1%) and adult patients without SARS-CoV-2 infection who served as controls from 34 medical centers nationwide between January 1, 2020, and December 7, 2020, patients were stratified using a World Health Organization COVID-19 severity scale and demographic characteristics. Differences between groups over time were evaluated using multivariable logistic regression. Random forest and XGBoost models were used to predict severe clinical course (death, discharge to hospice, invasive ventilatory support, or extracorporeal membrane oxygenation).
Main outcomes and measures: Patient demographic characteristics and COVID-19 severity using the World Health Organization COVID-19 severity scale and differences between groups over time using multivariable logistic regression.
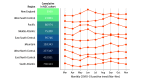
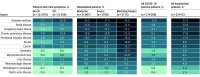
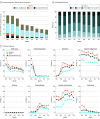
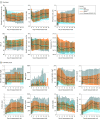
Results: The cohort included 174 568 adults who tested positive for SARS-CoV-2 (mean [SD] age, 44.4 [18.6] years; 53.2% female) and 1 133 848 adult controls who tested negative for SARS-CoV-2 (mean [SD] age, 49.5 [19.2] years; 57.1% female). Of the 174 568 adults with SARS-CoV-2, 32 472 (18.6%) were hospitalized, and 6565 (20.2%) of those had a severe clinical course (invasive ventilatory support, extracorporeal membrane oxygenation, death, or discharge to hospice). Of the hospitalized patients, mortality was 11.6% overall and decreased from 16.4% in March to April 2020 to 8.6% in September to October 2020 (P = .002 for monthly trend). Using 64 inputs available on the first hospital day, this study predicted a severe clinical course using random forest and XGBoost models (area under the receiver operating curve = 0.87 for both) that were stable over time. The factor most strongly associated with clinical severity was pH; this result was consistent across machine learning methods. In a separate multivariable logistic regression model built for inference, age (odds ratio [OR], 1.03 per year; 95% CI, 1.03-1.04), male sex (OR, 1.60; 95% CI, 1.51-1.69), liver disease (OR, 1.20; 95% CI, 1.08-1.34), dementia (OR, 1.26; 95% CI, 1.13-1.41), African American (OR, 1.12; 95% CI, 1.05-1.20) and Asian (OR, 1.33; 95% CI, 1.12-1.57) race, and obesity (OR, 1.36; 95% CI, 1.27-1.46) were independently associated with higher clinical severity.
Conclusions and relevance: This cohort study found that COVID-19 mortality decreased over time during 2020 and that patient demographic characteristics and comorbidities were associated with higher clinical severity. The machine learning models accurately predicted ultimate clinical severity using commonly collected clinical data from the first 24 hours of a hospital admission.
Conflict of interest statement
Figures
Update of
-
The National COVID Cohort Collaborative: Clinical Characterization and Early Severity Prediction.medRxiv [Preprint]. 2021 Jan 23:2021.01.12.21249511. doi: 10.1101/2021.01.12.21249511. medRxiv. 2021. Update in: JAMA Netw Open. 2021 Jul 1;4(7):e2116901. doi: 10.1001/jamanetworkopen.2021.16901. PMID: 33469592 Free PMC article. Updated. Preprint.
Comment in
-
Aggregating Electronic Health Record Data for COVID-19 Research-Caveat Emptor.JAMA Netw Open. 2021 Jul 1;4(7):e2117175. doi: 10.1001/jamanetworkopen.2021.17175. JAMA Netw Open. 2021. PMID: 34255055 No abstract available.
References
-
- Institutional Development Award Program Infrastructure for Clinical and Translational Research (IDeA-CTR). March 30, 2021. Accessed March 30, 2021. https://www.nigms.nih.gov/Research/DRCB/IDeA/Pages/IDeA-CTR.aspx
Publication types
MeSH terms
Grants and funding
- UL1 TR002014/TR/NCATS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 TR003017/TR/NCATS NIH HHS/United States
- UL1 TR002736/TR/NCATS NIH HHS/United States
- UL1 TR002553/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002544/TR/NCATS NIH HHS/United States
- U54 GM115516/GM/NIGMS NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR001876/TR/NCATS NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- U54 GM115428/GM/NIGMS NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- UL1 TR002645/TR/NCATS NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- UL1 TR002377/TR/NCATS NIH HHS/United States
- UL1 TR002733/TR/NCATS NIH HHS/United States
- UL1 TR002550/TR/NCATS NIH HHS/United States
- UL1 TR002529/TR/NCATS NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- U54 GM104941/GM/NIGMS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

