Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax
- PMID: 34255353
- PMCID: PMC10462434
- DOI: 10.1002/cncr.33689
Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax
Abstract
Background: TP53 mutation (TP53mut ) confers an adverse prognosis in acute myeloid leukemia (AML). Venetoclax with hypomethylating agents is a current standard for older patients; however, recent reports suggest that TP53mut confers resistance to venetoclax. The authors investigated the outcomes of patients with TP53mut AML who were treated with a 10-day decitabine and venetoclax (DEC10-VEN) (ClinicalTrials.gov identifier NCT03404193).
Methods: Patients with newly diagnosed AML received decitabine 20 mg/m2 for 10 days every 4 to 6 weeks for induction, followed by decitabine for 5 days after response. The venetoclax dose was 400 mg daily. TP53mut was identified in bone marrow samples using next-generation sequencing, with sensitivity of 5%. Outcomes were analyzed according to European LeukemiaNet 2017 guidelines.
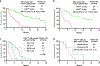
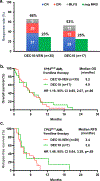
Results: Among 118 patients (median age, 72 years; age range, 49-89 years), 63 (53%) had secondary AML, 39 (33%) had AML with complex karyotype, and 35 (30%) had TP53mut AML. The median TP53 variant allele frequency was 32% (interquartile range, 16%-65%), 8 patients (23%) had only a single TP53 mutation, 15 (43%) had multiple mutations, and 12 (34%) had mutation and deletion. Outcomes were significantly worse in patients who had TP53mut AML compared with those who had wild-type TP53 AML, with an overall response rate of 66% vs 89% (P = .002), a complete response/complete response with incomplete hematologic recovery rate of 57% vs 77% (P = .029), and a 60-day mortality of 26% vs 4% (P < .001), respectively. Patients with TP53mut versus wild-type TP53 had shorter overall survival at 5.2 versus 19.4 months, respectively (hazard ratio, 4.67; 95% CI, 2.44-8.93; P < .0001), and shorter relapse-free survival at 3.4 versus 18.9 months (hazard ratio, 4.80; 95% CI, 1.97-11.69; P < .0001), respectively. Outcomes with DEC10-VEN in patients with TP53mut AML were comparable to historical results with 10-day decitabine alone.
Conclusions: Patients with TP53mut AML have lower response rates and shorter survival with DEC10-VEN.
Keywords: TP53; acute myeloid leukemia (AML); decitabine; outcome; venetoclax.
© 2021 American Cancer Society.
Conflict of interest statement
KK : None
AM : Celgene: Research Funding
SL : None
RP : None
TMK : Abbvie: Honoraria, Research Funding; Pulmotec: Research Funding; Celgene: Research Funding; Amgen: Research Funding; JAZZ: Honoraria, Research Funding; Cyclacel: Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Incyte: Research Funding; Astra Zeneca: Research Funding; Astellas: Research Funding; Novartis: Honoraria; Pfizer: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Cellenkos: Research Funding.
CRR : None
KF : None
ND : Gilead: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity’s Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Trovagene: Research Funding; Fate Therapeutics: Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding.
YA : None
MO : None
KS : None
NJS : Amgen: Honoraria; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Honoraria, Research Funding.
KT : Symbio Pharmaceuticals: Advisory board, Consultancy; GSK: Consultancy; Celgene: Consultancy
MY : Pint Pharma: Honoraria; Pfizer: Research Funding; Daicho Sankyo: Research Funding. Alvarado: Daiichi-Sankyo: Research Funding; BerGenBio ASA: Research Funding; MEI Pharma: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; Tolero Pharmaceuticals: Research Funding; FibroGen: Research Funding.
FR : Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Macrogenics: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding.
HMK : Daiichi-Sankyo: Honoraria, Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Abbvie: Honoraria, Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Actinium: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Delta Fly: Honoraria; Janssen: Honoraria; Oxford Biomedical: Honoraria; Immunogen: Research Funding.
CDD : Takeda: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; MedImmune: Honoraria; Notable Labs: Membership on an entity’s Board of Directors or advisory committees; Jazz: Honoraria; Agios: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy.
MYK : Amgen: Consultancy; Stemline Therapeutics: Consultancy, Research Funding; Agios: Research Funding; Calithera: Research Funding; Cellectis: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; AstraZeneca: Research Funding; Eli Lilly: Research Funding; Ablynx: Research Funding; Ascentage: Research Funding; Rafael Pharmaceutical: Research Funding; Kisoji: Consultancy; Sanofi: Research Funding; Forty-Seven: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding.
Figures

References
-
- Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 2009;23(1):203–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

