Cycloadditions with a Stable Charge-Separated Cyclobutadiene-Type Amido-Substituted Silicon Ring Compound
- PMID: 34255419
- PMCID: PMC8518912
- DOI: 10.1002/anie.202104341
Cycloadditions with a Stable Charge-Separated Cyclobutadiene-Type Amido-Substituted Silicon Ring Compound
Abstract
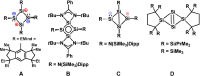
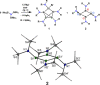
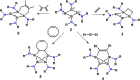
Reductive debromination of {N(SiMe3 )2 }SiBr3 with Rieke magnesium results in the formation of the five-vertex silicon cluster with one bromine substituent Si5 {N(SiMe3 )2 }5 Br, 1, and the cyclobutadiene analogue 2 in a 1:1 ratio. The latter features a planar four-membered silicon ring with a charge-separated electronic situation. Two silicon atoms in 2 are trigonal planar and the other two trigonal pyramidal. In cycloadditions with ethylene, diethylacetylene, 1,5-cyclooctadiene, and 2,3-dimethyl-1,3-butadiene cyclic unsaturated ring compounds (3-6) were formed at room temperature in quantitative reactions. Two of the products (3 and 6) show photochemical isomerization with LED light (λ=405 nm) to afford saturated ring compounds 4 e and 6'.
Keywords: charge separation; cycloaddition; photochemical isomerization; silicon amides; tetrasilacyclobutadiene.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

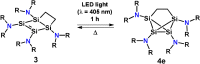
References
-
- Bally T., Angew. Chem. Int. Ed. 2006, 45, 6616–6619; - PubMed
- Angew. Chem. 2006, 118, 6768–6771.
-
- Wu J. I.-C., Mo Y., Evangelista F. A., Schleyer P. v. R., Chem. Commun. 2012, 48, 8437–8439. - PubMed
-
- Kostenko A., Tumanskii B., Kobayashi Y., Nakamoto M., Sekiguchi A., Apeloig Y., Angew. Chem. Int. Ed. 2017, 56, 10183–10187; - PubMed
- Angew. Chem. 2017, 129, 10317–10321.
-
- Emerson G. F., Watts L., Pettit R., J. Am. Chem. Soc. 1965, 87, 131–133.
-
- Pettit R., Henery J., Org. Synth. 1970, 50, 21.
Grants and funding
LinkOut - more resources
Full Text Sources

