Hyperglycemia Induces Trained Immunity in Macrophages and Their Precursors and Promotes Atherosclerosis
- PMID: 34255973
- PMCID: PMC8448412
- DOI: 10.1161/CIRCULATIONAHA.120.046464
Hyperglycemia Induces Trained Immunity in Macrophages and Their Precursors and Promotes Atherosclerosis
Abstract
Background: Cardiovascular risk in diabetes remains elevated despite glucose-lowering therapies. We hypothesized that hyperglycemia induces trained immunity in macrophages, promoting persistent proatherogenic characteristics.
Methods: Bone marrow-derived macrophages from control mice and mice with diabetes were grown in physiological glucose (5 mmol/L) and subjected to RNA sequencing (n=6), assay for transposase accessible chromatin sequencing (n=6), and chromatin immunoprecipitation sequencing (n=6) for determination of hyperglycemia-induced trained immunity. Bone marrow transplantation from mice with (n=9) or without (n=6) diabetes into (normoglycemic) Ldlr-/- mice was used to assess its functional significance in vivo. Evidence of hyperglycemia-induced trained immunity was sought in human peripheral blood mononuclear cells from patients with diabetes (n=8) compared with control subjects (n=16) and in human atherosclerotic plaque macrophages excised by laser capture microdissection.
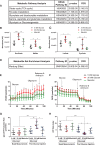
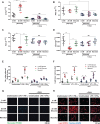
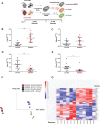
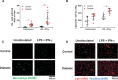
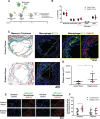
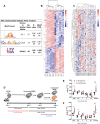
Results: In macrophages, high extracellular glucose promoted proinflammatory gene expression and proatherogenic functional characteristics through glycolysis-dependent mechanisms. Bone marrow-derived macrophages from diabetic mice retained these characteristics, even when cultured in physiological glucose, indicating hyperglycemia-induced trained immunity. Bone marrow transplantation from diabetic mice into (normoglycemic) Ldlr-/- mice increased aortic root atherosclerosis, confirming a disease-relevant and persistent form of trained innate immunity. Integrated assay for transposase accessible chromatin, chromatin immunoprecipitation, and RNA sequencing analyses of hematopoietic stem cells and bone marrow-derived macrophages revealed a proinflammatory priming effect in diabetes. The pattern of open chromatin implicated transcription factor Runt-related transcription factor 1 (Runx1). Similarly, transcriptomes of atherosclerotic plaque macrophages and peripheral leukocytes in patients with type 2 diabetes were enriched for Runx1 targets, consistent with a potential role in human disease. Pharmacological inhibition of Runx1 in vitro inhibited the trained phenotype.
Conclusions: Hyperglycemia-induced trained immunity may explain why targeting elevated glucose is ineffective in reducing macrovascular risk in diabetes and suggests new targets for disease prevention and therapy.
Keywords: diabetes mellitus; epigenetics; glucose; inflammation; macrophages.
Figures


Comment in
-
Hyperglycaemia-induced trained immunity promotes atherosclerosis.Nat Rev Cardiol. 2021 Oct;18(10):687. doi: 10.1038/s41569-021-00606-4. Nat Rev Cardiol. 2021. PMID: 34312499 No abstract available.
References
-
- American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2015; 38:S49–S57. doi: 10.2337/dc15-S011 - PubMed
-
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–2653. doi: 10.1056/NEJMoa052187 - PMC - PubMed
-
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986. doi: 10.1056/NEJM199309303291401 - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. doi: 10.1056/NEJMoa1504720 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

