Integrated vaccination and non-pharmaceutical interventions based strategies in Ontario, Canada, as a case study: a mathematical modelling study
- PMID: 34255985
- PMCID: PMC8277469
- DOI: 10.1098/rsif.2021.0009
Integrated vaccination and non-pharmaceutical interventions based strategies in Ontario, Canada, as a case study: a mathematical modelling study
Abstract
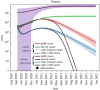
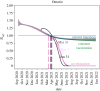
Recently, two coronavirus disease 2019 (COVID-19) vaccine products have been authorized in Canada. It is of crucial importance to model an integrated/combined package of non-pharmaceutical (physical/social distancing) and pharmaceutical (immunization) public health control measures. A modified epidemiological, compartmental SIR model was used and fit to the cumulative COVID-19 case data for the province of Ontario, Canada, from 8 September 2020 to 8 December 2020. Different vaccine roll-out strategies were simulated until 75% of the population was vaccinated, including a no-vaccination scenario. We compete these vaccination strategies with relaxation of non-pharmaceutical interventions. Non-pharmaceutical interventions were supposed to remain enforced and began to be relaxed on 31 January, 31 March or 1 May 2021. Based on projections from the data and long-term extrapolation of scenarios, relaxing the public health measures implemented by re-opening too early would cause any benefits of vaccination to be lost by increasing case numbers, increasing the effective reproduction number above 1 and thus increasing the risk of localized outbreaks. If relaxation is, instead, delayed and 75% of the Ontarian population gets vaccinated by the end of the year, re-opening can occur with very little risk. Relaxing non-pharmaceutical interventions by re-opening and vaccine deployment is a careful balancing act. Our combination of model projections from data and simulation of different strategies and scenarios, can equip local public health decision- and policy-makers with projections concerning the COVID-19 epidemiological trend, helping them in the decision-making process.
Keywords: COVID-19 pandemic; Canada; Ontario; mathematical modelling; non-pharmaceutical interventions; vaccine and immunization campaign.
Figures










References
-
- Government of Canada. The overall rollout approach. Canada's COVID-19 Immunization Plan: Saving Lives and Livelihoods. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coro... (accessed 29 December 2020).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

