Does the Reprocessing of Endoscopes Have to Take Place Immediately after Pre-Cleaning? A First Evaluation
- PMID: 34256556
- PMCID: PMC8357578
- DOI: 10.5946/ce.2020.238
Does the Reprocessing of Endoscopes Have to Take Place Immediately after Pre-Cleaning? A First Evaluation
Abstract
Background/aims: The recommendations on the time interval between pre-cleaning and reprocessing of endoscopes differ in international guidelines, with a low level of evidence. The aim of this study was to investigate the influence of postponing reprocessing on the reprocessing quality after pre-cleaning the flexible endoscopes.
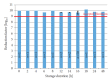
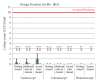
Methods: We reprocessed 124 standardized test tubes simulating endoscope channels after soiling and contamination and determined the reprocessing performance. In addition, we examined contaminated gastroscopes, colonoscopes, and bronchoscopes. The duration of interim storage after pre-cleaning was 16 h for 100 test tubes and up to 24 h for 18 endoscopes. We determined the residual protein content and germ load as markers for cleaning and disinfection performance. In addition, we determined biofilm formation by photometry of crystal violet staining.
Results: All test tubes and flexible endoscopes showed residual protein content and germ load significantly below legally prescribed threshold values, independent of the interval between pre-cleaning and reprocessing.
Conclusion: Our findings indicate that flexible endoscopes could be stored overnight after pre-cleaning without any influence on the quality of reprocessing. While ensuring patient safety, this could simplify logistical processes and enable cost savings.
Keywords: Biofilm; Endoscope; Pre-cleaning; Reprocessing; Validation.
Conflict of interest statement
Figures


Comment in
-
Endoscopes that Complete Pre-Cleaning may be Stored Overnight until Next Morning for the Subsequent Reprocessing.Clin Endosc. 2021 Jul;54(4):449-450. doi: 10.5946/ce.2021.135. Epub 2021 Jul 8. Clin Endosc. 2021. PMID: 34233404 Free PMC article. No abstract available.
References
-
- Kumarage J, Khonyongwa K, Khan A, Desai N, Hoffman P, Taori SK. Transmission of multi-drug resistant Pseudomonas aeruginosa between two flexible ureteroscopes and an outbreak of urinary tract infection: the fragility of endoscope decontamination. J Hosp Infect. 2019;102:89–94. - PubMed
-
- Naas T, Cuzon G, Babics A, et al. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2 beta-lactamase. J Antimicrob Chemother. 2010;65:1305–1306. - PubMed
-
- Shimono N, Takuma T, Tsuchimochi N, et al. An outbreak of Pseudomonas aeruginosa infections following thoracic surgeries occurring via the contamination of bronchoscopes and an automatic endoscope reprocessor. J Infect Chemother. 2008;14:418–423. - PubMed
-
- Shellnutt C. Advances in endoscope reprocessing technology and its impact on pathogen transmission. Gastroenterol Nurs. 2016;39:457–465. - PubMed
LinkOut - more resources
Full Text Sources

