Influence of Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in early-stage HER2 positive breast cancer
- PMID: 34257352
- PMCID: PMC8277791
- DOI: 10.1038/s41598-021-93634-6
Influence of Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in early-stage HER2 positive breast cancer
Abstract
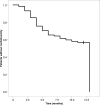
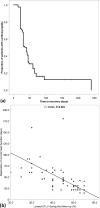
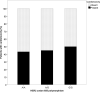
Trastuzumab has improved the prognosis of HER2 positive breast cancer, but cardiotoxicity remains a concern. We aimed to identify risk factors for trastuzumab-induced cardiotoxicity, with an emphasis on the HER2 Ile655Val single nucleotide polymorphism. This single-center case-control study included 1056 patients with early-stage HER2 positive breast cancer that received adjuvant trastuzumab. Cardiotoxicity was defined as a decline in left ventricular ejection fraction (LVEF) > 15% in patients without previous cardiomyopathy, or > 10% in patients with baseline LVEF of < 50%. Patient characteristics and cardiac parameters were compared in 78 (7.38%) cases and 99 randomly assigned controls, and the polymorphism was genotyped using real-time polymerase chain reaction. Cardiotoxicity was independently associated with advanced age (P = 0.024), lower body mass index (P = 0.023), left breast involvement (P = 0.001), N3 status (P = 0.004), diabetes (P = 0.016), and a family history of coronary artery disease (P = 0.019). Genotype distribution was as follows: A/A (Ile/Ile) was found in 111 (62.7%) patients, A/G (Ile/Val) in 60 (33.9%) patients, and G/G (Val/Val) in 6 (3.4%) patients. The genotype was not associated with cardiotoxicity or the severity of heart failure, reversibility, and recovery time. We found no association between the HER2 Ile655Val polymorphism and trastuzumab-induced cardiotoxicity; therefore, we do not recommend routine cardiotoxicity-risk stratification using this polymorphism.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

