Hybrid Capture-based Genomic Profiling of Circulating Tumor DNA From Patients With Advanced Ovarian Cancer
- PMID: 34257528
- PMCID: PMC8262155
- DOI: 10.3389/pore.2021.581534
Hybrid Capture-based Genomic Profiling of Circulating Tumor DNA From Patients With Advanced Ovarian Cancer
Abstract
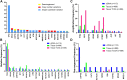
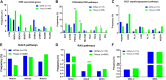
Objective: We conducted this study to characterize somatic genomic alterations in circulating tumor DNA (ctDNA) from patients with ovarian cancer and compare GAs detected in ctDNA with tissue databases. Methods: Hybrid capture-next generation sequencing genomic profiling of 150 genes was performed on ctDNA from 138 patients with ovarian cancer with 1,500× sequencing depth. The GAs detected in ctDNA were compared with those in our ovarian cancer tissue database (N = 488) and the Cancer Genome Atlas (TCGA) database (N = 489). Results: 115 patients (83%) had at least 1 GA detected in ctDNA. The most frequently altered genes detected in ctDNA were TP53 (72%), KRAS (11%), LRP1B (10%), ZNF703 (9%) and NF1 (8%). Comparative analysis with our tissue database showed similar frequencies of GAs per gene, although PIK3CA and KRAS mutations were more frequent in tissue and ctDNA, respectively (p < 0.05). Gene amplification and rearrangement were more frequent in ctDNA samples. The mutation frequency of homologous recombination repair associated-genes, VEGF signal/angiogenesis pathways, RAS pathways, NOTCH pathways and MSI-H ratio was not statistically different either in ctDNA or in tissue database. However, the mutation frequency of AKT, PIK3CA, PTEN and STK11 in PI3K/AKT/mTOR pathway was significantly lower than that in tissue samples (p < 0.05). Conclusions: Our results suggest that genomic profiling of ctDNA could detect somatic GAs in a significant subset of patients with ovarian cancer. Hybrid capture-NGS based on liquid biopsy has the potential capability to serve as a substitute to tissue biopsy and further studies are warranted.
Keywords: circulating tumor DNA; genomic alterations; genomic profiling; liquid biopsy; ovarian cancer.
Copyright © 2021 Shen, Shan, Liang, Zhang, Yu, Zhang, Wang, Bai, Qian, Lu and Jiang.
Conflict of interest statement
JZ, YY, YZ, GW, and YB were employed by the company 3D Medicines Inc., Shanghai, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer (version 1.2020). Available from: https://www.nccn.org/professionals/physician_gls/default.aspx. - PMC - PubMed
-
- Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov (2017). 7(9):984–98. 10.1158/2159-8290.cd-17-0419 - DOI - PMC - PubMed
-
- Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol (2019). 5(5):696–702. 10.1001/jamaoncol.2018.7098 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

