IMAT-IGRT Treatment with Simultaneous Integrated Boost as Dose Escalation for Patients with Cervical Cancer: A Single Institution, Prospective Pilot Study
- PMID: 34257570
- PMCID: PMC8262159
- DOI: 10.3389/pore.2021.608446
IMAT-IGRT Treatment with Simultaneous Integrated Boost as Dose Escalation for Patients with Cervical Cancer: A Single Institution, Prospective Pilot Study
Abstract
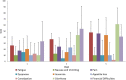
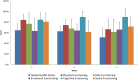
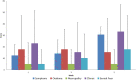
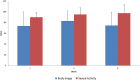
Purpose: The aim of this study was to introduce the simultaneous integrated boost (SIB) technique to assess the safety of replacement of the brachytherapy (BT) boost for ineligible patients with cervical cancer receiving radiochemotherapy (RCT). Methods: Fourteen patients were enrolled between 2015 and 2018. SIB was delivered using RapidArc technique at doses of 2.4 Gy per fraction during pelvic irradiation with 50.4/1.8 Gy in seven patients (to a total dose of 67.2 Gy) with limited volume disease. In 7 patients with a more advanced disease stage (>5 cm tumor, parametric invasion both sides), parametric boost therapy was added to the pelvic radiotherapy to a total dose of the macroscopic tumor of 79.2 Gy. All patients received simultaneous cisplatin-based chemotherapy for 5 cycles with a dosage of 40 mg/m2. We examined acute toxicity (CTCAE v4.1) and quality of life (EORTC QLQ30 and CX24). The tumor regression rate was evaluated with RECIST 1.1 after the first 3- to 4-months follow-up Magnetic Resonance Imaging (MRI) scan. We calculated the percentage of tumor regression rate and the local control during the follow-up period and evaluated the survival data. Results: Our patient data are presented at a median follow-up time of 24.5 months. During the treatment period, no grade 3 to 4 toxicity was observed. During the follow-up period, no late-onset toxicity was observed. The tumor regression rate at the first MRI scan was 95.31% on average. Disease free survival (DFS) during the median follow-up of 24 months was 98.6%. Conclusion: In patients with cervical cancer, the SIB technique is amenable as part of definitive RCT. Dose escalation with the SIB technique can be safely administered to cervical cancer patients during definitive RCT if BT is not feasible. However, further randomized clinical studies are needed to validate the method, so routine use of it cannot be recommended yet.
Keywords: IGRT; IMRT; SIB; cervical cancer; dose escalation; toxicity 3.
Copyright © 2021 Lőcsei, Sebestyén, Sebestyén, Fehér, Soltész, Musch and Mangel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kilic S, Cracchiolo B, Mahmoud O. Non-brachytherapy alternatives in cervical cancer radiotherapy: why not?. Appl Radiat Oncol (2015). 4(4):11–7.
-
- Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European society of gynaecological Oncology/European society for radiotherapy and Oncology/European society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol (2018). 127(3):404–16. 10.1016/j.radonc.2018.03.003 - DOI - PubMed
-
- Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, Miraldi F. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol (1999). 17:41. 10.1200/jco.1999.17.1.41 - DOI - PubMed

