Beneficial Effect of Lenalidomide-Dexamethason Treatment in Relapsed/Refractory Multiple Myeloma Patients: Results of Real-Life Data From 11 Hungarian Centers
- PMID: 34257583
- PMCID: PMC8262242
- DOI: 10.3389/pore.2021.613264
Beneficial Effect of Lenalidomide-Dexamethason Treatment in Relapsed/Refractory Multiple Myeloma Patients: Results of Real-Life Data From 11 Hungarian Centers
Abstract
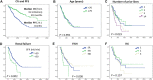
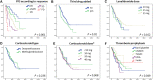
In Hungary, the cost of lenalidomide-based therapy is covered only for relapsed multiple myeloma (MM) patients, therefore lenalidomide is typically used in the second-line either as part of a triplet with proteasome inhibitors or as a doublet. Lenalidomide-dexamethasone is a standard treatment approach for relapsed/refractory MM, and according to recent large randomized clinical trials (RCT, the standard arm of POLLUX, ASPIRE, TOURMALINE), the progression-free survival (PFS) is expected to be approximately 18 months. We surveyed ten Hungarian centers treating MM and collected data of 278 patients treated predominantly after 2016. The median age was 65 years, and patients were distributed roughly equally over the 3 international staging system groups, but patients with high risk cytogenetics were underrepresented. 15.8% of the patients reached complete response, 21.6% very good partial response, 40.6% partial response, 10.8% stable disease, and 2.5% progressed on treatment. The median PFS was unexpectedly long, 24 months, however only 9 months in those with high risk cytogenetics. We found interesting differences between centers regarding corticosteroid type (prednisolone, methylprednisolone or dexamethasone) and dosing, and also regarding the choice of anticoagulation, but the outcome of the various centers were not different. Although the higher equivalent steroid dose resulted in more complete responses, the median PFS of those having lower corticosteroid dose and methylprednisolone were not inferior compared to the ones with higher dose dexamethasone. On multivariate analysis high risk cytogenetics and the number of prior lines remained significant independent prognostic factors regarding PFS (p < 0.001 and p = 0.005). Our results show that in well-selected patients Lenalidomide-dexamethasone can be a very effective treatment with real-world results that may even outperform those reported in the recent RCTs. This real world information may be more valuable than outdated RCT data when treatment options are discussed with patients.
Keywords: lenalidomide; myeloma; real life; relapsed; treatment.
Copyright © 2021 Varga, Dávid Tóth, Réka Szita, Csukly, Hardi, Gaál-Weisinger, Nagy, Altai, Rencsik, Plander, Szendrei, Kórád, Radványi, Rottek, Deák, Szaleczky, Schneider, Kohl, Kosztolányi, Alizadeh, Lengyel, Modok, Borbényi, Lovas, Váróczy, Illés, Rajnics, Masszi and Mikala.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, et al. Long-term Follow-Up on Overall Survival from the MM-009 and MM-010 Phase III Trials of Lenalidomide Plus Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. Leukemia (2009) 23:2147–52. 10.1038/leu.2009.147 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

