Efficacy of minute virus of mice (MVM) inactivation utilizing high temperature short time (HTST) pasteurization and suitability assessment of pasteurized, concentrated glucose feeds in Chinese hamster ovary (CHO) cell expression systems
- PMID: 34257631
- PMCID: PMC8257999
- DOI: 10.1002/elsc.202100044
Efficacy of minute virus of mice (MVM) inactivation utilizing high temperature short time (HTST) pasteurization and suitability assessment of pasteurized, concentrated glucose feeds in Chinese hamster ovary (CHO) cell expression systems
Abstract
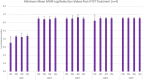
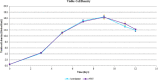
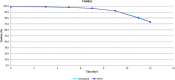
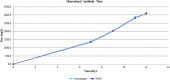
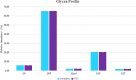
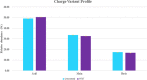
There is a growing need to provide effective adventitious agent mitigation for high risk upstream cell culture raw materials used for the production of biologics. It is also highly important in the growing fields of cell and gene therapies. Glucose is a critical raw material necessary for effective cell growth and productivity; however, glucose is the highest risk animal-origin-free raw material for viral contamination, and often the highest risk raw material in the upstream process as more companies move to chemically defined media. This study examines the efficacy of utilizing High Temperature Short Time (HTST) pasteurization for inactivation of physiochemically resistant, worst-case parvovirus using a bench-scale HTST system. We demonstrated approximately six log inactivation of Minute Virus of Mice (MVM) in concentrated glucose feeds without impacting the subsequent performance of the glucose in a Chinese Hamster Ovary (CHO) expression system.
Keywords: high temperature short time; minute virus of mice; pasteurization; upstream cell culture risk mitigation.
© 2021 Merck KGaA. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest. Authors acknowledge that MilliporeSigma offer HTST‐treated glucose feeds at commercial‐scale volumes; however, this is not viewed as a conflict of interest as there are other vendors in the marketplace offering similar services. In addition, the treatment conditions recommended in this paper can be implemented by any organization on their internal HTST systems.
Figures

References
-
- Peter Kosiol, B. H. , Ulbricht, M. , Thom, V. , Determination of pore size distributions of virus filtration membranes using gold nanoparticles and their correlation with virus retention. J Membrane Sci 2017, 533, 289–301.
-
- Shah, K. , Nathanson, N. , Human exposure to SV40: review and comment. Am J Epidemiol 1976, 103, 1–12. - PubMed
-
- Barone, P. W. , Wiebe, M. E. , Leung, J. C. , Hussein, I. T. M. , et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat Biotechnol 2020, 38, 563–572. - PubMed
LinkOut - more resources
Full Text Sources
