Catalytic (3 + 2) annulation of donor-acceptor aminocyclopropane monoesters and indoles
- PMID: 34257869
- PMCID: PMC8246098
- DOI: 10.1039/d1sc01127h
Catalytic (3 + 2) annulation of donor-acceptor aminocyclopropane monoesters and indoles
Abstract
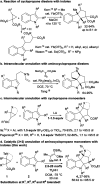
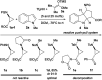
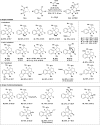
The efficient catalytic activation of donor-acceptor aminocyclopropanes lacking the commonly used diester acceptor is reported here in a (3 + 2) dearomative annulation with indoles. Bench-stable tosyl-protected aminocyclopropyl esters were converted into cycloadducts in 46-95% yields and up to 95 : 5 diastereomeric ratio using catalytic amounts of triethylsilyl triflimide. Tricyclic indoline frameworks containing four stereogenic centers including all-carbon quaternary centers were obtained.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
-
Selected reviews:
- Reissig H.-U. Zimmer R. Chem. Rev. 2003;103:1151–1196. - PubMed
- Yu M. Pagenkopf B. L. Tetrahedron. 2005;61:321.
- Cavitt M. A. Phun L. H. France S. Chem. Soc. Rev. 2014;43:804–818. - PubMed
- Schneider T. F. Kaschel J. Werz D. B. Angew. Chem., Int. Ed. 2014;53:5504–5523. - PubMed
- Grover H. K. Emmett M. R. Kerr M. A. Org. Biomol. Chem. 2015;13:655–671. - PubMed
- Pandey A. K. Ghosh A. Banerjee P. Isr. J. Chem. 2016;56:512–521.
- Talukdar R. Saha A. Ghorai M. K. Isr. J. Chem. 2016;56:445–453.
- Reiser O. Isr. J. Chem. 2016;56:531–539.
- Budynina E. M. Ivanov K. L. Sorokin I. D. Melnikov M. Y. Synthesis. 2017;49:3035–3068.
-
-
-
Selected examples:
- Pohlhaus P. D. Johnson J. S. J. Am. Chem. Soc. 2005;127:16014–16015. - PubMed
- Pohlhaus P. D. Johnson J. S. J. Org. Chem. 2005;70:1057–1059. - PubMed
- Parsons A. T. Johnson J. S. J. Am. Chem. Soc. 2009;131:3122–3123. - PubMed
- Parsons A. T. Smith A. G. Neel A. J. Johnson J. S. J. Am. Chem. Soc. 2010;132:9688–9692. - PubMed
- Carson C. A. Kerr M. A. J. Org. Chem. 2005;70:8242–8244. - PubMed
- Curiel Tejeda J. E. Irwin L. C. Kerr M. A. Org. Lett. 2016;18:4738–4741. - PubMed
- Qu J.-P. Deng C. Zhou J. Sun X.-L. Tang Y. J. Org. Chem. 2009;74:7684–7689. - PubMed
- Xu H. Qu J.-P. Liao S. Xiong H. Tang Y. Angew. Chem., Int. Ed. 2013;52:4004–4007. - PubMed
- Xiong H. Xu H. Liao S. Xie Z. Tang Y. J. Am. Chem. Soc. 2013;135:7851–7854. - PubMed
- Yang G. Shen Y. Li K. Sun Y. Hua Y. J. Org. Chem. 2011;76:229–233. - PubMed
- Sathishkannan G. Srinivasan K. Org. Lett. 2011;13:6002–6005. - PubMed
- Goldberg A. F. G. Connor N. R. O. Craig R. A. Stoltz B. M. Org. Lett. 2012;14:5314–5317. - PMC - PubMed
- Wang H. Yang W. Liu H. Wang W. Li H. Org. Biomol. Chem. 2012;10:5032–5035. - PubMed
- Chakrabarty S. Chatterjee I. Wibbeling B. Daniliuc C. G. Studer A. Angew. Chem., Int. Ed. 2014;53:5964–5968. - PubMed
- Wang D.-C. Xie M.-S. Guo H.-M. Qu G.-R. Zhang M.-C. You S.-L. Angew. Chem., Int. Ed. 2016;55:14111–14115. - PubMed
- Verma K. Banerjee P. Adv. Synth. Catal. 2016;358:2053–2058.
- Augustin A. U. Sensse M. Jones P. G. Werz D. B. Angew. Chem., Int. Ed. 2017;56:14293–14296. - PubMed
- Augustin A. U. Busse M. Jones P. G. Werz D. B. Org. Lett. 2018;20:820–823. - PubMed
- Ahlburg N. L. Jones P. G. Werz D. B. Org. Lett. 2020;22:6404–6408. - PubMed
- Kaga A. Gandamana D. A. Tamura S. Demirelli M. Chiba S. Synlett. 2017;28:1091–1095.
- Matsumoto Y. Nakatake D. Yazaki R. Ohshima T. Chem.–Eur. J. 2018;24:6062–6066. - PubMed
- Mondal M. Panda M. Davis N. W. McKee V. Kerrigan N. J. Chem. Commun. 2019;55:13558–13561. - PubMed
-
-
-
For a review see:
- de Nanteuil F. De Simone F. Frei R. Benfatti F. Serrano E. Waser J. Chem. Commun. 2014;50:10912–10928. - PubMed
-
; selected examples:
- de Nanteuil F. Waser J. Angew. Chem., Int. Ed. 2011;50:12075–12079. - PubMed
- Benfatti F. de Nanteuil F. Waser J. Org. Lett. 2012;14:386–389. - PubMed
- Benfatti F. de Nanteuil F. Waser J. Chem.–Eur. J. 2012;18:4844–4849. - PubMed
- Racine S. de Nanteuil F. Serrano E. Waser J. Angew. Chem., Int. Ed. 2014;53:8484–8487. - PubMed
- de Nanteuil F. Serrano E. Perrotta D. Waser J. J. Am. Chem. Soc. 2014;136:6239–6242. - PubMed
- Racine S. Hegedüs B. Scopelliti R. Waser J. Chem.–Eur. J. 2016;22:11997–12001. - PubMed
- Preindl J. Chakrabarty S. Waser J. Chem. Sci. 2017;8:7112–7118. - PMC - PubMed
- Suleymanov A. A. Le Du E. Dong Z. Muriel B. Scopelliti R. Fadaei-Tirani F. Waser J. Severin K. Org. Lett. 2020;22:4517–4522. - PubMed
- Rivero A. R. Fernandez I. Sierra M. Org. Lett. 2013;15:4928–4931. - PubMed
- Zhang M.-C. Wang D.-C. Xie M.-S. Qu G.-R. Guo H.-M. You S.-L. Chem. 2019;5:156–167.
- Hao E.-J. Fu D.-D. Wang D.-C. Zhang T. Qu G.-R. Li G.-X. Lan Y. Guo H.-M. Org. Chem. Front. 2019;6:863–867.
- Wang H.-X. Li W.-P. Zhang M.-M. Xie M.-S. Qu G.-R. Guo H.-M. Chem. Commun. 2020;56:11649–11652. - PubMed
-
-
- Zhang P.-P. Yan Z.-M. Li Y.-H. Gong J.-X. Yang Z. J. Am. Chem. Soc. 2017;139:13989–13992. - PubMed
LinkOut - more resources
Full Text Sources

