Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Host Displaying Treatment Induced Viral Evolution
- PMID: 34258320
- PMCID: PMC8244814
- DOI: 10.1093/ofid/ofab295
Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Host Displaying Treatment Induced Viral Evolution
Abstract
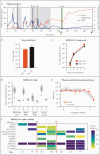
We report a coronavirus disease 2019 case with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persisting beyond 333 days in an immunocompromised patient with chronic lymphocytic leukemia, asymptomatically carrying infectious SARS-CoV-2 at day 197 postdiagnosis. In addition, viral sequencing indicates major changes in the spike protein over time, temporally associated with convalescent plasma treatment.
Keywords: CLL; SARS-CoV-2; persistent infection; viral evolution.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584:115–9. - PubMed
-
- Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–62. - PubMed
-
- Centers for Disease Control and Prevention. Discontinuation of isolation for persons with COVID-19 not in healthcare settings. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patien.... Accessed 4 February 2021.
LinkOut - more resources
Full Text Sources
Miscellaneous

