Early life administration of milk fat globule membrane promoted SCFA-producing bacteria colonization, intestinal barriers and growth performance of neonatal piglets
- PMID: 34258422
- PMCID: PMC8245794
- DOI: 10.1016/j.aninu.2020.07.012
Early life administration of milk fat globule membrane promoted SCFA-producing bacteria colonization, intestinal barriers and growth performance of neonatal piglets
Abstract
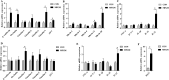
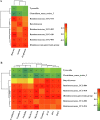
Milk fat globule membrane (MFGM) possesses various nutritional and biological benefits for mammals, whereas its effects on neonatal gut microbiota and barrier integrity remained unclear. This study investigated the effects of MFGM administration on microbial compositions and intestinal barrier functions of neonatal piglets. Sixteen newborn piglets were randomly allocated into a CON group or MFGM group, orally administered with saline or MFGM solution (1 g/kg body weight) respectively during the first postnatal week, and all piglets were breastfed during the whole neonatal period. The present study found that the MFGM oral administration during the first postnatal week increased the plasma immunoglobulin (Ig) G level, body weight and average daily gain of piglets (P < 0.05) on 21 d. Additionally, MFGM administration enriched fecal SCFA-producing bacteria (Ruminococ aceae_UCG-002, Ruminococ aceae_UCG-010, Ruminococ aceae_UCG-004, Ruminococ aceae_UCG-014 and [Ruminococcus]_gauvrearuii_group), SCFA concentrations (acetate, propionate and butyrate; P < 0.05) and their receptor (G-protein coupled receptor 41, GPR41). Furthermore, MFGM administration promoted intestinal villus morphology (P < 0.05) and barrier functions by upregulating genes of tight junctions (E-cadherin, claudin-1, occludin and zonula occludin 1 [ZO-1]), mucins (mucin-13 and mucin-20) and interleukin (IL)-22 (P < 0.05). Positive correlation was found between the beneficial microbes and SCFA levels pairwise with the intestinal barrier genes (P < 0.05). In conclusion, orally administrating MFGM during the first postnatal week stimulated SCFA-producing bacteria colonization and SCFA generation, enhanced intestinal barrier functions and consequently improved growth performance of neonatal piglets on 21 d. Our findings will provide new insights about MFGM intervention for microbial colonization and intestinal development of neonates during their early life.
Keywords: Gut microbiota; Intestinal barrier; Milk fat globule membrane; Piglet; SCFA.
© 2021 Chinese Association of Animal Science and Veterinary Medicine. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Figures




References
-
- Ageitos J.M., Sanchez-Perez A., Calo-Mata P., Villa T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017;133:117–118. - PubMed
-
- Atroshi F., Alaviuhkola T., Schildt R., Sandholm M. Fat globule-membrane of sow milk as a target for adhesion of K88-positive Escherichia-coli. Comp Immunol Microb. 1983;6(3):235–245. - PubMed
LinkOut - more resources
Full Text Sources

