Skin-on-a-chip models: General overview and future perspectives
- PMID: 34258497
- PMCID: PMC8270645
- DOI: 10.1063/5.0046376
Skin-on-a-chip models: General overview and future perspectives
Erratum in
-
Erratum: Publisher's Note: "Skin-on-a-chip models: General overview and future perspectives" [APL Bioengineering 5, 030901 (2021)].APL Bioeng. 2021 Oct 14;5(4):049901. doi: 10.1063/5.0073964. eCollection 2021 Dec. APL Bioeng. 2021. PMID: 34703971 Free PMC article.
Abstract
Over the last few years, several advances have been made toward the development and production of in vitro human skin models for the analysis and testing of cosmetic and pharmaceutical products. However, these skin models are cultured under static conditions that make them unable to accurately represent normal human physiology. Recent interest has focused on the generation of in vitro 3D vascularized skin models with dynamic perfusion and microfluidic devices known as skin-on-a-chip. These platforms have been widely described in the literature as good candidates for tissue modeling, as they enable a more physiological transport of nutrients and permit a high-throughput and less expensive evaluation of drug candidates in terms of toxicity, efficacy, and delivery. In this Perspective, recent advances in these novel platforms for the generation of human skin models under dynamic conditions for in vitro testing are reported. Advances in vascularized human skin equivalents (HSEs), transferred skin-on-a-chip (introduction of a skin biopsy or a HSE in the chip), and in situ skin-on-a-chip (generation of the skin model directly in the chip) are critically reviewed, and currently used methods for the introduction of skin cells in the microfluidic chips are discussed. An outlook on current applications and future directions in this field of research are also presented.
© 2021 Author(s).
Figures
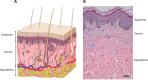




References
-
- Bensouilah J. and Buck P., “ Skin: Structure and function,” in Aromadermatology ( CRC Press, 2016), pp. 1–10.
-
- Mescher A. L., Junqueira's Basic Histology, 13th ed. ( Text & Atlas, 2013).
-
- Kolarsick P. A. J., Kolarsick M. A., and Goodwin C., “ Anatomy and physiology of the skin,” J. Dermatol. Nurses Assoc. 3(4), 203–213 (2011).10.1097/JDN.0b013e3182274a98 - DOI
LinkOut - more resources
Full Text Sources

