Bioengineering platforms for cell therapeutics derived from pluripotent and direct reprogramming
- PMID: 34258498
- PMCID: PMC8263070
- DOI: 10.1063/5.0040621
Bioengineering platforms for cell therapeutics derived from pluripotent and direct reprogramming
Abstract
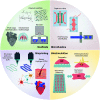
Pluripotent and direct reprogramming technologies hold great potential for tissue repair and restoration of tissue and organ function. The implementation of induced pluripotent stem cells and directly reprogrammed cells in biomedical research has resulted in a significant leap forward in the highly promising area of regenerative medicine. While these therapeutic strategies are promising, there are several obstacles to overcome prior to the introduction of these therapies into clinical settings. Bioengineering technologies, such as biomaterials, bioprinting, microfluidic devices, and biostimulatory systems, can enhance cell viability, differentiation, and function, in turn the efficacy of cell therapeutics generated via pluripotent and direct reprogramming. Therefore, cellular reprogramming technologies, in combination with tissue-engineering platforms, are poised to overcome current bottlenecks associated with cell-based therapies and create new ways of producing engineered tissue substitutes.
© 2021 Author(s).
Figures

Similar articles
-
Bioengineering strategies to accelerate stem cell therapeutics.Nature. 2018 May;557(7705):335-342. doi: 10.1038/s41586-018-0089-z. Epub 2018 May 16. Nature. 2018. PMID: 29769665 Free PMC article. Review.
-
Concise review: reprogramming strategies for cardiovascular regenerative medicine: from induced pluripotent stem cells to direct reprogramming.Stem Cells Transl Med. 2014 Apr;3(4):448-57. doi: 10.5966/sctm.2013-0163. Epub 2014 Mar 3. Stem Cells Transl Med. 2014. PMID: 24591731 Free PMC article. Review.
-
Recent advances in stem cell therapeutics and tissue engineering strategies.Biomater Res. 2018 Dec 19;22:36. doi: 10.1186/s40824-018-0148-4. eCollection 2018. Biomater Res. 2018. PMID: 30598836 Free PMC article. Review.
-
Advances in Regenerative Medicine and Biomaterials.Methods Mol Biol. 2023;2575:127-152. doi: 10.1007/978-1-0716-2716-7_7. Methods Mol Biol. 2023. PMID: 36301474
-
Robust Differentiation of mRNA-Reprogrammed Human Induced Pluripotent Stem Cells Toward a Retinal Lineage.Stem Cells Transl Med. 2016 Apr;5(4):417-26. doi: 10.5966/sctm.2015-0093. Epub 2016 Mar 1. Stem Cells Transl Med. 2016. PMID: 26933039 Free PMC article.
Cited by
-
Acoustofluidic bioassembly induced morphogenesis for therapeutic tissue fabrication.Nat Commun. 2025 May 5;16(1):4174. doi: 10.1038/s41467-025-59026-4. Nat Commun. 2025. PMID: 40324975 Free PMC article.
-
Functional biomaterials.APL Bioeng. 2022 Jan 3;6(1):010401. doi: 10.1063/5.0078930. eCollection 2022 Mar. APL Bioeng. 2022. PMID: 34993383 Free PMC article. No abstract available.
-
Updated Perspectives on Direct Vascular Cellular Reprogramming and Their Potential Applications in Tissue Engineered Vascular Grafts.J Funct Biomater. 2022 Dec 30;14(1):21. doi: 10.3390/jfb14010021. J Funct Biomater. 2022. PMID: 36662068 Free PMC article. Review.
-
Durable Muscle Extracellular Matrix Engineered with Adhesive Phenolic Moieties for Effective Skeletal Muscle Regeneration in Muscle Atrophy.Adv Healthc Mater. 2024 Dec;13(32):e2401826. doi: 10.1002/adhm.202401826. Epub 2024 Oct 17. Adv Healthc Mater. 2024. PMID: 39420690 Free PMC article.
-
Advanced lung organoids for respiratory system and pulmonary disease modeling.J Tissue Eng. 2024 Feb 22;15:20417314241232502. doi: 10.1177/20417314241232502. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38406820 Free PMC article. Review.
References
-
- Mirbagheri M., Adibnia V., Hughes B. R., Waldman S. D., Banquy X., and Hwang D. K., Mater. Horiz. 6(1), 45–71 (2019).10.1039/C8MH00803E - DOI
Publication types
LinkOut - more resources
Full Text Sources

