Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy
- PMID: 34258584
- PMCID: PMC8266040
- DOI: 10.1158/2643-3230.BCD-21-0038
Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy
Erratum in
-
Correction: Tumor Burden Limits Bispecific Antibody Efficacy through T-cell Exhaustion Averted by Concurrent Cytotoxic Therapy.Blood Cancer Discov. 2022 Jan;3(1):82. doi: 10.1158/2643-3230.BCD-21-0176. Blood Cancer Discov. 2022. PMID: 35015694 Free PMC article. No abstract available.
Abstract
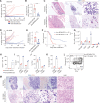
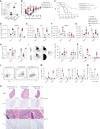
BCMA-CD3-targeting bispecific antibodies (BsAb) are a recently developed immunotherapy class which shows potent tumor killing activity in multiple myeloma (MM). Here, we investigated a murine BCMA-CD3-targeting BsAb in the immunocompetent Vk*MYC and its IMiD-sensitive derivative Vk*MYChCRBN models of MM. The BCMA-CD3 BsAb was safe and efficacious in a subset of mice, but failed in those with high-tumor burden, consistent with clinical reports of BsAb in leukemia. The combination of BCMA-CD3 BsAb with pomalidomide expanded lytic T cells and improved activity even in IMiD resistant high-tumor burden cases. Yet, survival was only marginally extended due to acute toxicity and T cell exhaustion, which impaired T cell persistence. In contrast, the combination with cyclophosphamide was safe and allowed for a tempered pro-inflammatory response associated with long-lasting complete remission. Concurrent cytotoxic therapy with BsAb actually improved T cell persistence and function, offering a promising approach to patients with a large tumor burden.
Figures




References
-
- Chesi M, Fonseca R.Antibodies create killer bonds in myeloma. Cancer Cell 2017;31:305–7. - PubMed
-
- Costa LJ, Wong SW, Bermúdez A, de la Rubia J, Mateos MV, Ocio EM, et al. First clinical study of the B-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Blood 2019;134:143.
-
- Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Evaluation of AMG 420, an anti-BCMA bispecific T cell engager (BiTE) immunotherapy, in R/R multiple myeloma (MM) patients: updated results of a first-in-human (FIH) phase I dose escalation study. J Clin Oncol 2019;37:8007.
-
- Garfall AL, Usmani SZ, Mateos MV, Nahi H, van de Donk NWCJ, San-Miguel JF, et al. Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed and/or refractory multiple myeloma (RRMM). Blood 2020;136:27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

