Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: Results from a real-world study in South Korea
- PMID: 34258882
- PMCID: PMC8419762
- DOI: 10.1002/cam4.4127
Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: Results from a real-world study in South Korea
Abstract
Objectives: The optimal sequence for the administration of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for treating non-small cell lung cancer (NSCLC) is still unclear. This study aimed to evaluate the efficacy of sequential afatinib and osimertinib treatment in patients with NSCLC harboring EGFR mutations.
Materials and methods: Electronic records of patients with EGFR-mutated NSCLC, who were administered afatinib and osimertinib (group A) or other chemotherapy (group B) between October 2014 and 2019, across 16 hospitals in South Korea were reviewed. The primary outcome, time on treatment (TOT), secondary outcome, and overall survival (OS) were estimated using the Kaplan-Meier method and log-rank test. Multivariate analyses were performed using the Cox proportional hazards model.
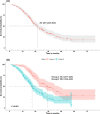
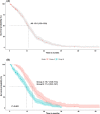
Results: Of the 737 patients who received frontline afatinib treatment, 324 with complete records were selected (group A: 126, group B: 198). All patients in group A were T790M positive after afatinib, while patients in group B were all negative or unknown. The median TOT was 35.4 months (95% confidence interval [CI]: 27.7-45.6) in group A and 20.8 months (95% CI: 19.4-24.0) in group B. The median TOT with afatinib was 13.0 months (95% CI: 12.0-13.9) overall and 15.7 months (95% CI: 13.9-17.3) in group A. The 2- and 3-year survival rates were 86.0 and 69.3% in group A and 75.9 and 55.3% in group B, respectively.
Conclusion: Sequential afatinib and osimertinib treatment resulted in better survival rates than treatment with afatinib followed by other chemotherapies.
Keywords: EGFR; NSCLC; afatinib; osimertinib; real-world data.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
no conflict of interest.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. - PubMed
-
- Park K, Tan E‐H, O'Byrne K, et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): a phase 2B, open‐label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

