Critically Ill Patients Treated for Chimeric Antigen Receptor-Related Toxicity: A Multicenter Study
- PMID: 34259446
- PMCID: PMC8678137
- DOI: 10.1097/CCM.0000000000005149
Critically Ill Patients Treated for Chimeric Antigen Receptor-Related Toxicity: A Multicenter Study
Abstract
Objectives: To report the epidemiology, treatments, and outcomes of adult patients admitted to the ICU after cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome.
Design: Retrospective cohort study.
Setting: Nine centers across the U.S. part of the chimeric antigen receptor-ICU initiative.
Patients: Adult patients treated with chimeric antigen receptor T-cell therapy who required ICU admission between November 2017 and May 2019.
Interventions: Demographics, toxicities, specific interventions, and outcomes were collected.
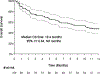
Results: One-hundred five patients treated with axicabtagene ciloleucel required ICU admission for cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome during the study period. At the time of ICU admission, the majority of patients had grade 3-4 toxicities (66.7%); 15.2% had grade 3-4 cytokine release syndrome and 64% grade 3-4 immune effector cell-associated neurotoxicity syndrome. During ICU stay, cytokine release syndrome was observed in 77.1% patients and immune effector cell-associated neurotoxicity syndrome in 84.8% of patients; 61.9% patients experienced both toxicities. Seventy-nine percent of patients developed greater than or equal to grade 3 toxicities during ICU stay, however, need for vasopressors (18.1%), mechanical ventilation (10.5%), and dialysis (2.9%) was uncommon. Immune Effector Cell-Associated Encephalopathy score less than 3 (69.7%), seizures (20.2%), status epilepticus (5.7%), motor deficits (12.4%), and cerebral edema (7.9%) were more prevalent. ICU mortality was 8.6%, with only three deaths related to cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome. Median overall survival time was 10.4 months (95% CI, 6.64-not available mo). Toxicity grade or organ support had no impact on overall survival; higher cumulative corticosteroid doses were associated to decreased overall and progression-free survival.
Conclusions: This is the first study to describe a multicenter cohort of patients requiring ICU admission with cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. Despite severe toxicities, organ support and in-hospital mortality were low in this patient population.
Copyright © 2021 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
Conflict of interest statement
This data was accepted to be presented as abstracts and an oral presentation at the Society of Critical Care Medicine Congress in Orlando, FL, 2020. Drs. Gutierrez, Brudno, and Athale received support for article research from the National Institutes of Health. Dr. Gutierrez disclosed the off-label product use of anakinra; she disclosed that she served, and will serve again, in the advisory board for Legend Biotech and Janssen in August 2020. Drs. Gutierrez, May, and McEvoy disclosed the off-label product use of Siltuximab. Dr. Brown received funding from La Jolla Pharmaceutical outside the submitted work. Dr. May disclosed the off-label product use of Corticosteroids. Dr. Beitinjaneh received funding from KITE pharmaceuticals on August 2018. Drs. Brudno and Kochenderfer disclosed government work. Dr. Kochenderfer’s institution received funding from KITE pharmaceuticals, Bristol Meyers Squibb, and Kyverna; he is the principal investigator of Cooperative Research and Development Agreements with Kite, a Gilead Company and Celgene. Dr. Lin as Principal Investigator Mayo Clinic receives compensation for research activities and clinical trials with Kite/Gilead, Janssen, Celgene, BlueBird Bio, Merck, Boston Scientific, Gamida, and Takeda; advisory board with Kite/Gilead, Novartis, Janssen, Legend BioTech, JUNO, Bristol-Myers-Squibb (BMS), Celgene, BlueBird Bio, and Ethos; Data and Safety Monitoring Board: Sorrento; and steering committee: Celgene, Janssen, and Legend BioTech. Dr. McEvoy received funding from United Therapeutics. Dr. Westin received funding from BMS, Novartis, Kite Gilead, Juno Celgene, Genentech, AstraZeneca, Morphosys, and ADC Therapeutics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


Comment in
-
Chimeric Antigen Receptor T Cells, the Shock of the New.Crit Care Med. 2022 Jan 1;50(1):157-160. doi: 10.1097/CCM.0000000000005153. Crit Care Med. 2022. PMID: 34914647 No abstract available.
References
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

