Perinatal Outcomes of Two Screening Strategies for Gestational Diabetes Mellitus: A Randomized Controlled Trial
- PMID: 34259458
- PMCID: PMC8288467
- DOI: 10.1097/AOG.0000000000004431
Perinatal Outcomes of Two Screening Strategies for Gestational Diabetes Mellitus: A Randomized Controlled Trial
Abstract
Objective: To evaluate differences in short-term perinatal outcomes between the two prominent screening strategies for gestational diabetes mellitus, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) and Carpenter-Coustan.
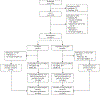
Methods: In this single-site, blinded, randomized, comparative effectiveness trial, participants received a nonfasting 50-g oral glucose tolerance test and, if less than 200 mg/dL (less than 11.1 mmol/L), were randomized to further screening with either IADPSG or Carpenter-Coustan criteria. Gestational diabetes treatment occurred per routine clinical care. The primary outcome was incidence of large-for-gestational-age (LGA) neonates. Prespecified secondary outcomes included small-for-gestational-age (SGA) neonates, cesarean birth, and neonatal and maternal composites of adverse perinatal outcomes. Assuming a 15% incidence of LGA neonates in the Carpenter-Coustan group, 782 participants provided more than 80% power to detect a 7% absolute risk reduction with the use of IADPSG; planned recruitment was 920 for anticipated attrition.
Results: From June 2015 to February 2019, 1,016 participants were enrolled and 921 were randomized to IADPSG (n=461) or Carpenter-Coustan (n=460) groups. Gestational diabetes incidence (14.4% vs 4.5%, P<.001) and diabetes medication use (9.3% vs 2.4%; P<.001) were more common in the IADPSG group; there were no differences in LGA neonates, either overall (risk reduction 0.90, 97.5% CI 0.53-1.52) or among women without gestational diabetes (risk reduction 0.85, 97.5% CI 0.49-1.48). Those screened with IADPSG had higher rates of neonatal morbidity but fewer study-related adverse events. Rates of SGA neonates, cesarean birth, and maternal morbidity composite did not differ significantly between study groups.
Conclusions: The IADPSG screening criteria resulted in more women diagnosed and treated for gestational diabetes than Carpenter-Coustan without reducing the incidence of LGA birth weight or maternal or neonatal morbidity.
Clinical trial registration: ClinicalTrials.gov, NCT02309138.
Copyright © 2021 by the American College of Obstetricians and Gynecologists. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure The authors did not report any potential conflicts of interest.
Figures
Comment in
-
Gestational Diabetes Mellitus Screening Strategies: Balancing Short-term and Long-term Risks.Obstet Gynecol. 2021 Jul 1;138(1):3-5. doi: 10.1097/AOG.0000000000004453. Obstet Gynecol. 2021. PMID: 34259457 No abstract available.
-
Perinatal Outcomes of Two Screening Strategies for Gestational Diabetes Mellitus.Obstet Gynecol. 2021 Oct 1;138(4):680-681. doi: 10.1097/AOG.0000000000004553. Obstet Gynecol. 2021. PMID: 34623083 No abstract available.
References
-
- Yang J, Cummings EA, O’Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstetrics and gynecology. 2006;108(3 Pt 1):644–650. - PubMed
-
- Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstetrics and gynecology. 1997;90(6):869–873. - PubMed
-
- Sibai BM, Gordon T, Thom E, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172(2 Pt 1):642–648. - PubMed
-
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117(5):575–584. - PubMed
-
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

