Liver X receptor β regulates bile volume and the expression of aquaporins and cystic fibrosis transmembrane conductance regulator in the gallbladder
- PMID: 34259574
- PMCID: PMC8815792
- DOI: 10.1152/ajpgi.00024.2021
Liver X receptor β regulates bile volume and the expression of aquaporins and cystic fibrosis transmembrane conductance regulator in the gallbladder
Abstract
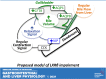
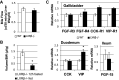
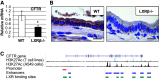
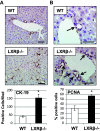
The gallbladder is considered an important organ in maintaining digestive and metabolic homeostasis. Given that therapeutic options for gallbladder diseases are often limited to cholecystectomy, understanding gallbladder pathophysiology is essential in developing novel therapeutic strategies. Since liver X receptor β (LXRβ), an oxysterol-activated transcription factor, is strongly expressed in gallbladder cholangiocytes, the aim was to investigate LXRβ physiological function in the gallbladder. Thus, we studied the gallbladders of WT and LXRβ-/- male mice using immunohistochemistry, electron microscopy, qRT-PCR, bile duct cannulation, bile and blood biochemistry, and duodenal pH measurements. LXRβ-/- mice presented a large gallbladder bile volume with high duodenal mRNA levels of the vasoactive intestinal polypeptide (VIP), a strong mediator of gallbladder relaxation. LXRβ-/- gallbladders showed low mRNA and protein expression of Aquaporin-1, Aquaporin-8, and cystic fibrosis transmembrane conductance regulator (CFTR). A cystic fibrosis-resembling phenotype was evident in the liver showing high serum cholestatic markers and the presence of reactive cholangiocytes. For LXRβ being a transcription factor, we identified eight putative binding sites of LXR on the promoter and enhancer of the Cftr gene, suggesting Cftr as a novel LXRβ regulated gene. In conclusion, LXRβ was recognized as a regulator of gallbladder bile volume through multiple mechanisms involving CFTR and aquaporins.NEW & NOTEWORTHY This report reveals a novel and specific role of the nuclear receptor liver X receptor β (LXRβ) in controlling biliary tree pathophysiology. LXRβ-/- mice have high gallbladder bile volume and are affected by a cholangiopathy that resembles cystic fibrosis. We found LXRβ to regulate the expression of both aquaporins water channels and the cystic fibrosis transmembrane conductance regulator. This opens a new field in biliary tree pathophysiology, enlightening a possible transcription factor controlling CFTR expression.
Keywords: CFTR; aquaporins; cholangiocytes; gallbladder; nuclear receptors.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl MA. Novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14: 7025–7035, 1994. doi: 10.1128/MCB.14.10.7025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

