Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer's disease
- PMID: 34259835
- PMCID: PMC8677538
- DOI: 10.1093/brain/awab223
Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer's disease
Abstract
Although recent clinical trials targeting amyloid-β in Alzheimer's disease have shown promising results, there is increasing evidence suggesting that understanding alternative disease pathways that interact with amyloid-β metabolism and amyloid pathology might be important to halt the clinical deterioration. In particular, there is evidence supporting a critical role of astroglial activation and astrocytosis in Alzheimer's disease. However, so far, no studies have assessed whether astrocytosis is independently related to either amyloid-β or tau pathology in vivo. To address this question, we determined the levels of the astrocytic marker GFAP in plasma and CSF of 217 amyloid-β-negative cognitively unimpaired individuals, 71 amyloid-β-positive cognitively unimpaired individuals, 78 amyloid-β-positive cognitively impaired individuals, 63 amyloid-β-negative cognitively impaired individuals and 75 patients with a non-Alzheimer's disease neurodegenerative disorder from the Swedish BioFINDER-2 study. Participants underwent longitudinal amyloid-β (18F-flutemetamol) and tau (18F-RO948) PET as well as cognitive testing. We found that plasma GFAP concentration was significantly increased in all amyloid-β-positive groups compared with participants without amyloid-β pathology (P < 0.01). In addition, there were significant associations between plasma GFAP with higher amyloid-β-PET signal in all amyloid-β-positive groups, but also in cognitively normal individuals with normal amyloid-β values (P < 0.001), which remained significant after controlling for tau-PET signal. Furthermore, plasma GFAP could predict amyloid-β-PET positivity with an area under the curve of 0.76, which was greater than the performance achieved by CSF GFAP (0.69) and other glial markers (CSF YKL-40: 0.64, soluble TREM2: 0.71). Although correlations were also observed between tau-PET and plasma GFAP, these were no longer significant after controlling for amyloid-β-PET. In contrast to plasma GFAP, CSF GFAP concentration was significantly increased in non-Alzheimer's disease patients compared to other groups (P < 0.05) and correlated with amyloid-β-PET only in amyloid-β-positive cognitively impaired individuals (P = 0.005). Finally, plasma GFAP was associated with both longitudinal amyloid-β-PET and cognitive decline, and mediated the effect of amyloid-β-PET on tau-PET burden, suggesting that astrocytosis secondary to amyloid-β aggregation might promote tau accumulation. Altogether, these findings indicate that plasma GFAP is an early marker associated with brain amyloid-β pathology but not tau aggregation, even in cognitively normal individuals with a normal amyloid-β status. This suggests that plasma GFAP should be incorporated in current hypothetical models of Alzheimer's disease pathogenesis and be used as a non-invasive and accessible tool to detect early astrocytosis secondary to amyloid-β pathology.
Keywords: Aβ-PET; GFAP; astrocytosis; cognition; tau-PET.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


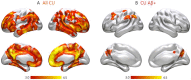
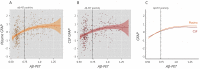


References
-
- Osborn LM, Kamphuis W, Wadman WJ, Hol EM.. Astrogliosis: An integral player in the pathogenesis of Alzheimer's disease. Prog Neurobiol. 2016;144:121–141. - PubMed
-
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D.. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunology. 1989;24(3):173–182. - PubMed
-
- Beach TG, McGeer EG.. Lamina-specific arrangement of astrocytic gliosis and senile plaques in Alzheimer's disease visual cortex. Brain Res. 1988;463(2):357–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

