The challenge of choosing in cardiovascular risk management
- PMID: 34259995
- PMCID: PMC8724475
- DOI: 10.1007/s12471-021-01599-y
The challenge of choosing in cardiovascular risk management
Abstract
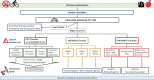
Cardiovascular disease (CVD) is a major cause of morbidity and mortality worldwide. For many years guidelines have listed optimal preventive therapy. More recently, novel therapeutic options have broadened the options for state-of-the-art CV risk management (CVRM). In the majority of patients with CVD, risk lowering can be achieved by utilising standard preventive medication combined with lifestyle modifications. In a minority of patients, add-on therapies should be considered to further reduce the large residual CV risk. However, the choice of which drug combination to prescribe and in which patients has become increasingly complicated, and is dependent on both the absolute CV risk and the reason for the high risk. In this review, we discuss therapeutic decisions in CVRM, focusing on (1) the absolute CV risk of the patient and (2) the pros and cons of novel treatment options.
Keywords: Atherosclerosis; Cardiovascular disease; Cardiovascular risk management; Drugs; Novel interventions; Prevention.
© 2021. The Author(s).
Conflict of interest statement
N.M.J. Hanssen reports personal fees from Ingelheim Boehringer. A. Mosterd reports grants from Novartis, and personal fees from Amarin, Amgen, BMS, Boehringer Ingelheim, MSD and Pfizer, outside the scope of the submitted work. C.J. Tack reports grants and personal fees from NovoNordisk, personal fees from MSD, Bayer and Boehringer Ingelheim, grants from AstraZeneca, the European Foundation for the Study of Diabetes, funding from the Perspectief Biomarker Development Center Research Programme, which is (partly) financed by the Netherlands Organisation for Scientific Research (NWO), and funding from the Innovative Medicines Initiative (IMI) consortium HypoResolve, funded jointly by the European Union and the European pharmaceutical industry (EFPIA), during the conduct of the study. J. R. van Lennep reports personal fees from Medcon, during the conduct of the study; and grants from the Dutch Heart Foundation and Amryt, outside the scope of the submitted work. J.H. Cornel reports grants from ZonMw and the Hartstichting, and personal fees from Amgen and AstraZeneca, outside the scope of the submitted work. J.W. Jukema reports that his department has received research grants from and/or was speaker (with or without lecture fees) at (CME accredited) meetings sponsored by Amgen, Athera, AstraZeneca, Biotronik, Boston Scientific, Dalcor, Daiichi Sankyo, Lilly, Medtronic, Merck-Schering-Plough, Pfizer, Roche, Sanofi Aventis, The Medicine Company, the Netherlands Heart Foundation, CardioVascular Research in the Netherlands (CVON), the Netherlands Heart Institute and the European Community Framework KP7 Programme, during the conduct of the study. E.S.G. Stroes reports personal fees paid to the institution by Amgen, Sanofi-Regeneron, Novartis, Akcea, Esperion, Novo-Nordisk and Athera, all outside the scope of the submitted work; and the Netherlands Heart Foundation (CVON-GENIUS-II) as well as the EU-HORIZON (REPROGRAM; ERA-CVD). R.M. Hoogeveen, J.R. Brouwer, A.A. (Bram) Kroon, G.J. de Borst, J. ten Berg, T. van Trier, A. Liem, E. Serné, F.L.J. Visseren and R.J.G. Peters the PANORAMA working group declare that they have no competing interests.
Figures
References
-
- Jaspers NEM, Ridker PM, Dorresteijn JAN, Visseren FLJ. The prediction of therapy-benefit for individual cardiovascular disease prevention: rationale, implications, and implementation. Curr Opin Lipidol. 2018;29:436–444. - PubMed
-
- Dorresteijn JAN, Visseren FLJ, Wassink AMJ, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99:866–872. - PubMed
-
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. - PubMed
-
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

