A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice
- PMID: 34260064
- PMCID: PMC9292003
- DOI: 10.1111/nph.17619
A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice
Abstract
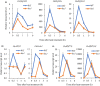
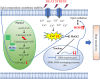
Heat stress is a major environmental threat affecting crop growth and productivity. However, the molecular mechanisms associated with plant responses to heat stress are poorly understood. Here, we identified a heat stress-sensitive mutant, hts1, in rice. HTS1 encodes a thylakoid membrane-localized β-ketoacyl carrier protein reductase (KAR) involved in de novo fatty acid biosynthesis. Phylogenetic and bioinformatic analysis showed that HTS1 probably originated from streptophyte algae and is evolutionarily conserved in land plants. Thermostable HTS1 is predominantly expressed in green tissues and strongly induced by heat stress, but is less responsive to salinity, cold and drought treatments. An amino acid substitution at A254T in HTS1 causes a significant decrease in KAR enzymatic activity and, consequently, impairs fatty acid synthesis and lipid metabolism in the hts1 mutant, especially under heat stress. Compared to the wild-type, the hts1 mutant exhibited heat-induced higher H2 O2 accumulation, a larger Ca2+ influx to mesophyll cells, and more damage to membranes and chloroplasts. Also, disrupted heat stress signaling in the hts1 mutant depresses the transcriptional activation of HsfA2s and the downstream target genes. We suggest that HTS1 is critical for underpinning membrane stability, chloroplast integrity and stress signaling for heat tolerance in rice.
Keywords: Oryza sativa; heat; hydrogen peroxide; lipids; membrane; signaling.
© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Figures




Similar articles
-
HTT1, a Stearoyl-Acyl Carrier Protein Desaturase Involved Unsaturated Fatty Acid Biosynthesis, Affects Rice Heat Tolerance.Plant Cell Environ. 2025 May;48(5):3391-3405. doi: 10.1111/pce.15359. Epub 2025 Jan 5. Plant Cell Environ. 2025. PMID: 39757551
-
Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance.BMC Plant Biol. 2016 Sep 20;16(1):203. doi: 10.1186/s12870-016-0897-y. BMC Plant Biol. 2016. PMID: 27646344 Free PMC article.
-
OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2 O2 signalling in rice.Plant Biotechnol J. 2017 Feb;15(2):183-196. doi: 10.1111/pbi.12601. Epub 2016 Nov 11. Plant Biotechnol J. 2017. PMID: 27420922 Free PMC article.
-
Biological Roles of Lipids in Rice.Int J Mol Sci. 2024 Aug 21;25(16):9046. doi: 10.3390/ijms25169046. Int J Mol Sci. 2024. PMID: 39201734 Free PMC article. Review.
-
Drought and heat stress mediated activation of lipid signaling in plants: a critical review.Front Plant Sci. 2023 Aug 10;14:1216835. doi: 10.3389/fpls.2023.1216835. eCollection 2023. Front Plant Sci. 2023. PMID: 37636093 Free PMC article. Review.
Cited by
-
Modulation of warm temperature-sensitive growth using a phytochrome B dark reversion variant, phyB[G515E], in Arabidopsis and rice.J Adv Res. 2024 Sep;63:57-72. doi: 10.1016/j.jare.2023.11.001. Epub 2023 Nov 4. J Adv Res. 2024. PMID: 37926145 Free PMC article.
-
Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress.Int J Mol Sci. 2021 Nov 15;22(22):12308. doi: 10.3390/ijms222212308. Int J Mol Sci. 2021. PMID: 34830190 Free PMC article. Review.
-
Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis.Plants (Basel). 2025 Apr 1;14(7):1072. doi: 10.3390/plants14071072. Plants (Basel). 2025. PMID: 40219140 Free PMC article.
-
TTLOC: A Tn5 transposase-based approach to localize T-DNA integration sites.Plant Physiol. 2025 Mar 28;197(4):kiaf102. doi: 10.1093/plphys/kiaf102. Plant Physiol. 2025. PMID: 40131780 Free PMC article.
-
Responses of the tree peony (Paeonia suffruticosa, Paeoniaceae) cultivar 'Yu Hong' to heat stress revealed by iTRAQ-based quantitative proteomics.Proteome Sci. 2022 Dec 29;20(1):18. doi: 10.1186/s12953-022-00202-5. Proteome Sci. 2022. PMID: 36578066 Free PMC article.
References
-
- Balogh G, Peter M, Glatz A, Gombos I, Torok Z, Horvath I, Harwood JL, Vigh L. 2013. Key role of lipids in heat stress management. FEBS Letters 587: 1970–1980. - PubMed
-
- Bao X, Focke M, Pollard M, Ohlrogge J. 2000. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. The Plant Journal 22: 39–50. - PubMed
-
- Barkla BJ, Pantoja O. 2011. Plasma membrane and abiotic stress. Berlin, Germany: Springer.
-
- Bergler H, Wallner P, Ebeling A, Leitinger B, Fuchsbichler S, Aschauer H, Kollenz G, Hogenauer G, Turnowsky F. 1994. Protein EnvM is the NADH‐dependent enoyl‐ACP reductase (FabI) of Escherichia coli . Journal of Biological Chemistry 269: 5493–5496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

