Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy
- PMID: 34260074
- PMCID: PMC8457117
- DOI: 10.1002/mus.27356
Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy
Abstract
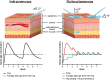
Immunoglobulin G (IgG) therapy is an established long-term treatment in chronic inflammatory demyelinating polyneuropathy (CIDP) that is commonly administered intravenously (IVIg). The subcutaneous immunoglobulin (SCIg) administration route is a safe and effective alternative option, approved by the United States Food and Drug Administration (FDA) in 2018, for maintenance treatment of adults with CIDP. Physicians and patients alike need to be aware of all their treatment options in order to make informed decisions and plan long-term treatment strategies. In this review, we collate the evidence for SCIg in CIDP from all published studies and discuss their implications and translation to clinical practice. We also provide guidance on the practicalities of how and when to transition patients from IVIg to SCIg and ongoing patient support. Evidence suggests that IVIg and SCIg have comparable long-term efficacy in CIDP. However, SCIg can provide additional benefits for some patients, including no requirement for venous access or premedication, and reduced frequency of systemic adverse events. Local-site reactions are more common with SCIg than IVIg, but these are mostly well-tolerated and abate with subsequent infusions. Data suggest that many patients prefer SCIg following transition from IVIg. SCIg preference may be a result of the independence and flexibility associated with self-infusion, whereas IVIg preference may be a result of familiarity and reliance on a healthcare professional for infusions. In practice, individualizing maintenance dosing based on disease behavior and determining the minimally effective IgG dose for individuals are key considerations irrespective of the administration route chosen.
Keywords: CIDP; IVIg; SCIg; immunoglobulin therapy; transition.
© 2021 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
Mazen Dimachkie serves, or recently served, as a consultant for ArgenX, Catalyst, Cello, CSL Behring, EcoR1, Kezar, Momenta, NuFactor, Octapharma, RaPharma/UCB, RMS Medical, Sanofi Genzyme, Shire Takeda, Spark Therapeutics and UCB Biopharma. Dr. Dimachkie received grants from Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol‐Myers Squibb, Catalyst, Corbus, CSL‐Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, UCB Biopharma, Viromed/Healixmith & TMA. Namita Goyal has received research support from Brainstorm Cell Therapeutics, Cytokinetics, Fulcrum, Kezar, Octapharma, Orion, Orphazyme. Dr. Goyal has served on Advisory Boards for Acceleron, Alexion, Argenx, Biogen, CSL Behring, Cytokinetics, MT Pharma, Sanofi Genzyme, Sarepta. In relation to these activities, she has received travel reimbursement and honoraria. She has also served on the speaker's bureau for CSL. The remaining authors have no conflicts of interest. Kazim Sheikh has received personal compensation for speaking engagements from CSL Behring and research/grant support from the Department of Defense (W81XWH‐18‐1‐0422) and the National Institute of Neurological Disorders and Stroke (R21NS107961). Chafic Karam has consulted for Acceleron Pharma, Inc; Akcea Therapeutics; Alnylam Pharmaceuticals, Inc; Argenx; Biogen; CSL Behring; and Sanofi Genzyme. Dr Karam has received personal compensation for speaking engagements from Akcea Therapeutics; Alnylam Pharmaceuticals, Inc; CSL Behring; and Sanofi Genzyme and research/grant support from Akcea Therapeutics and Sanofi Genzyme.
Figures

References
-
- Lamb YN, Syed YY, Dhillon S. Immune globulin subcutaneous (human) 20% (Hizentra[®]): a review in chronic inflammatory demyelinating polyneuropathy. CNS Drugs. 2019;33(8):831‐838. - PubMed
-
- Gelinas D, Katz J, Nisbet P, England JD. Current practice patterns in CIDP: a cross‐sectional survey of neurologists in the United States. J Neurol Sci. 2019;397:84‐91. - PubMed
-
- Gorson KC, van Schaik IN, Merkies ISJ, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst. 2010;15(4):326‐333. - PubMed

