Toward a Multipathway Perspective: pH-Dependent Kinetic Selection of Competing Pathways and the Role of the Internal Glutamate in Cl-/H+ Antiporters
- PMID: 34260231
- PMCID: PMC8409247
- DOI: 10.1021/acs.jpcb.1c03304
Toward a Multipathway Perspective: pH-Dependent Kinetic Selection of Competing Pathways and the Role of the Internal Glutamate in Cl-/H+ Antiporters
Abstract
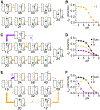
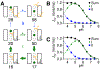
Canonical descriptions of multistep biomolecular transformations generally follow a single-pathway viewpoint, with a series of transitions through intermediates converting reactants to products or repeating a conformational cycle. However, mounting evidence suggests that more complexity and pathway heterogeneity are mechanistically relevant due to the statistical distribution of multiple interconnected rate processes. Making sense of such pathway complexity remains a significant challenge. To better understand the role and relevance of pathway heterogeneity, we herein probe the chemical reaction network of a Cl-/H+ antiporter, ClC-ec1, and analyze reaction pathways using multiscale kinetic modeling (MKM). This approach allows us to describe the nature of the competing pathways and how they change as a function of pH. We reveal that although pH-dependent Cl-/H+ transport rates are largely regulated by the charge state of amino acid E148, the charge state of E203 determines relative contributions from coexisting pathways and can shift the flux pH-dependence. The selection of pathways via E203 explains how ionizable mutations (D/H/K/R) would impact the ClC-ec1 bioactivity from a kinetic perspective and lends further support to the indispensability of an internal glutamate in ClC antiporters. Our results demonstrate how quantifying the kinetic selection of competing pathways under varying conditions leads to a deeper understanding of the Cl-/H+ exchange mechanism and can suggest new approaches for mechanistic control.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Jentsch TJ; Stein V; Weinreich F; Zdebik AA Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev 2002, 82, 503–568. - PubMed
-
- Jentsch TJ; Pusch M CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev 2018, 98, 1493–1590. - PubMed
-
- Miller C ClC Chloride Channels Viewed through a Transporter Lens. Nature 2006, 440, 484–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

