SARS-CoV-2 Disrupts Proximal Elements in the JAK-STAT Pathway
- PMID: 34260266
- PMCID: PMC8428404
- DOI: 10.1128/JVI.00862-21
SARS-CoV-2 Disrupts Proximal Elements in the JAK-STAT Pathway
Abstract
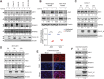
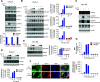
SARS-CoV-2 can infect multiple organs, including lung, intestine, kidney, heart, liver, and brain. The molecular details of how the virus navigates through diverse cellular environments and establishes replication are poorly defined. Here, we generated a panel of phenotypically diverse, SARS-CoV-2-infectible human cell lines representing different body organs and performed longitudinal survey of cellular proteins and pathways broadly affected by the virus. This revealed universal inhibition of interferon signaling across cell types following SARS-CoV-2 infection. We performed systematic analyses of the JAK-STAT pathway in a broad range of cellular systems, including immortalized cells and primary-like cardiomyocytes, and found that SARS-CoV-2 targeted the proximal pathway components, including Janus kinase 1 (JAK1), tyrosine kinase 2 (Tyk2), and the interferon receptor subunit 1 (IFNAR1), resulting in cellular desensitization to type I IFN. Detailed mechanistic investigation of IFNAR1 showed that the protein underwent ubiquitination upon SARS-CoV-2 infection. Furthermore, chemical inhibition of JAK kinases enhanced infection of stem cell-derived cultures, indicating that the virus benefits from inhibiting the JAK-STAT pathway. These findings suggest that the suppression of interferon signaling is a mechanism widely used by the virus to evade antiviral innate immunity, and that targeting the viral mediators of immune evasion may help block virus replication in patients with COVID-19. IMPORTANCE SARS-CoV-2 can infect various organs in the human body, but the molecular interface between the virus and these organs remains unexplored. In this study, we generated a panel of highly infectible human cell lines originating from various body organs and employed these cells to identify cellular processes commonly or distinctly disrupted by SARS-CoV-2 in different cell types. One among the universally impaired processes was interferon signaling. Systematic analysis of this pathway in diverse culture systems showed that SARS-CoV-2 targets the proximal JAK-STAT pathway components, destabilizes the type I interferon receptor though ubiquitination, and consequently renders the infected cells resistant to type I interferon. These findings illuminate how SARS-CoV-2 can continue to propagate in different tissues even in the presence of a disseminated innate immune response.
Keywords: IFN antagonism; IFN signaling; JAK-STAT pathway; SARS-CoV-2; human cell lines; immune evasion; proteomics; virus-host interactions.
Figures




References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, Elsoukkary S, He B, Del Vecchio C, Fortarezza F, Pezzuto F, Navalesi P, Crisanti A, Fowkes ME, Bryce CH, Calabrese F, Beasley MB. 2020. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol 33:2156–2168. 10.1038/s41379-020-00661-1. - DOI - PMC - PubMed
-
- Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. 2020. SARS-CoV-2 productively infects human gut enterocytes. Science 369:50–54. 10.1126/science.abc1669. - DOI - PMC - PubMed
-
- Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. 2020. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 73:807–816. 10.1016/j.jhep.2020.05.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI085089/AI/NIAID NIH HHS/United States
- NIH R01HL132325/HHS | National Institutes of Health (NIH)
- Peter Paul Career Development Award
- National Science Foundation Nanosystems Engineering Research Center for Directed Multiscale Assembly of Cellular Metamaterials
- R01 CA175382/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

