A Semiautomated Luciferase Immunoprecipitation Assay for Rapid and Easy Detection of African Swine Fever Virus Antibody
- PMID: 34260273
- PMCID: PMC8451409
- DOI: 10.1128/JCM.00990-21
A Semiautomated Luciferase Immunoprecipitation Assay for Rapid and Easy Detection of African Swine Fever Virus Antibody
Abstract
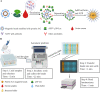
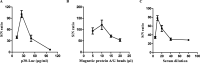
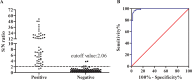
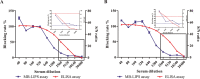
African swine fever (ASF) is a highly contagious viral disease of domestic pigs and wild boars. For disease surveillance and control, we developed a rapid and easy luciferase immunoprecipitation assay (MB-LIPS) to detect ASF virus (ASFV) antibody. The MB-LIPS is based on magnetic beads modified with protein A/G and the recombinant fusion protein of ASFV p30 and luciferase, where p30 functioned as the recognition element and luciferase as the signal component. Incubation and washing could be finished automatically on a machine with magnetic rods. Under optimal conditions, the MB-LIPS showed 96.3% agreement to a commercial enzyme-linked immunosorbent assay (ELISA) kit for detecting ASFV antibody in swine sera. Analyzing serial dilutions of a swine serum sample showed that the MP-LIPS assay was 4 times more sensitive than the ELISA kit. The whole run of the MB-LIPS could be completed within 30 min. With its high sensitivity and simple operation, the MB-LIPS platform has great potential to be used for the detection of ASFV antibody and ASF control in small labs and farms.
Keywords: African swine fever virus; luciferase immunoprecipitation system; magnetic beads; rapid; sensitivity.
Figures

Similar articles
-
Development of a highly sensitive Gaussia luciferase immunoprecipitation assay for the detection of antibodies against African swine fever virus.Front Cell Infect Microbiol. 2022 Sep 14;12:988355. doi: 10.3389/fcimb.2022.988355. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189357 Free PMC article.
-
An immunoassay based on bioluminescent sensors for rapid detection of African swine fever virus antibodies.J Clin Microbiol. 2024 Oct 16;62(10):e0046324. doi: 10.1128/jcm.00463-24. Epub 2024 Sep 5. J Clin Microbiol. 2024. PMID: 39235247 Free PMC article.
-
One-Step Rapid and Sensitive ASFV p30 Antibody Detection via Nanoplasmonic Biosensors.Microbiol Spectr. 2022 Dec 21;10(6):e0234322. doi: 10.1128/spectrum.02343-22. Epub 2022 Oct 31. Microbiol Spectr. 2022. PMID: 36314937 Free PMC article.
-
[African swine fever in Russian Federation].Vopr Virusol. 2012 Sep-Oct;57(5):4-10. Vopr Virusol. 2012. PMID: 23248852 Review. Russian.
-
African swine fever in the North Caucasus region and the Russian Federation in years 2007-2012.Virus Res. 2013 Apr;173(1):198-203. doi: 10.1016/j.virusres.2012.12.007. Epub 2012 Dec 22. Virus Res. 2013. PMID: 23266725 Review.
Cited by
-
Evaluating the clinical utility of semi-quantitative luciferase immunosorbent assay using Treponema pallidum antigens in syphilis diagnosis and treatment monitoring.Emerg Microbes Infect. 2024 Dec;13(1):2348525. doi: 10.1080/22221751.2024.2348525. Epub 2024 May 16. Emerg Microbes Infect. 2024. PMID: 38661428 Free PMC article.
-
Advanced Strategies for Developing Vaccines and Diagnostic Tools for African Swine Fever.Viruses. 2023 Oct 28;15(11):2169. doi: 10.3390/v15112169. Viruses. 2023. PMID: 38005846 Free PMC article. Review.
-
Development of plate-type and tubular chemiluminescence immunoassay against African swine fever virus p72.Appl Microbiol Biotechnol. 2024 Aug 2;108(1):431. doi: 10.1007/s00253-024-13249-5. Appl Microbiol Biotechnol. 2024. PMID: 39093478 Free PMC article.
-
Advancing Luciferase-Based Antibody Immunoassays to Next-Generation Mix and Read Testing.Biosensors (Basel). 2023 Feb 21;13(3):303. doi: 10.3390/bios13030303. Biosensors (Basel). 2023. PMID: 36979515 Free PMC article. Review.
-
Development of practical techniques for simultaneous detection and distinction of current and emerging SARS-CoV-2 variants.Anal Sci. 2023 Nov;39(11):1839-1856. doi: 10.1007/s44211-023-00396-4. Epub 2023 Jul 30. Anal Sci. 2023. PMID: 37517003 Review.
References
-
- O’Donnell VK, Grau FR, Mayr GA, Sturgill Samayoa TL, Dodd KA, Barrette RW. 2019. Rapid sequence-based characterization of African swine fever virus by use of the Oxford Nanopore MinION sequence sensing device and a companion analysis software tool. J Clin Microbiol 58:e01104-19. 10.1128/JCM.01104-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

