Preventing microalbuminuria with benazepril, valsartan, and benazepril-valsartan combination therapy in diabetic patients with high-normal albuminuria: A prospective, randomized, open-label, blinded endpoint (PROBE) study
- PMID: 34260595
- PMCID: PMC8279302
- DOI: 10.1371/journal.pmed.1003691
Preventing microalbuminuria with benazepril, valsartan, and benazepril-valsartan combination therapy in diabetic patients with high-normal albuminuria: A prospective, randomized, open-label, blinded endpoint (PROBE) study
Abstract
Background: Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) prevent microalbuminuria in normoalbuminuric type 2 diabetic patients. We assessed whether combined therapy with the 2 medications may prevent microalbuminuria better than ACE inhibitor or ARB monotherapy.
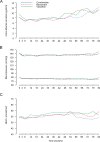
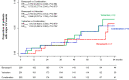
Methods and findings: VARIETY was a prospective, randomized, open-label, blinded endpoint (PROBE) trial evaluating whether, at similar blood pressure (BP) control, combined therapy with benazepril (10 mg/day) and valsartan (160 mg/day) would prevent microalbuminuria more effectively than benazepril (20 mg/day) or valsartan (320 mg/day) monotherapy in 612 type 2 diabetic patients with high-normal albuminuria included between July 2007 and April 2013 by the Istituto di Ricerche Farmacologiche Mario Negri IRCCS and 8 diabetology or nephrology units in Italy. Time to progression to microalbuminuria was the primary outcome. Analyses were intention to treat. Baseline characteristics were similar among groups. During a median [interquartile range, IQR] follow-up of 66 [42 to 83] months, 53 patients (27.0%) on combination therapy, 57 (28.1%) on benazepril, and 64 (31.8%) on valsartan reached microalbuminuria. Using an accelerated failure time model, the estimated acceleration factors were 1.410 (95% CI: 0.806 to 2.467, P = 0.229) for benazepril compared to combination therapy, 0.799 (95% CI: 0.422 to 1.514, P = 0.492) for benazepril compared to valsartan, and 1.665 (95% CI: 1.007 to 2.746, P = 0.047) for valsartan compared to combination therapy. Between-group differences in estimated acceleration factors were nonsignificant after adjustment for predefined confounders. BP control was similar across groups. All treatments were safe and tolerated well, with a slight excess of hyperkalemia and hypotension in the combination therapy group. The main study limitation was the lower than expected albuminuria at inclusion.
Conclusions: Risk/benefit profile of study treatments was similar. Dual renin-angiotensin system (RAS) blockade is not recommended as compared to benazepril or valsartan monotherapy for prevention of microalbuminuria in normoalbuminuric type 2 diabetic patients.
Trial registration: EudraCT 2006-005954-62; ClinicalTrials.gov NCT00503152.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: GR is a member of PLOS Medicine’s Editorial Board. All other authors declare no competing interests.
Figures


References
-
- Ruggenenti P, Trillini M, P Barlovic D, Cortinovis M, Pisani A, Parvanova A, et al.. Effects of valsartan, benazepril and their combination in overt nephropathy of type 2 diabetes: A prospective, randomized, controlled trial. Diabetes Obes Metab. 2019;21:1177–90. doi: 10.1111/dom.13639 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

