Evaluating Vaccine Efficacy Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection
- PMID: 34260716
- PMCID: PMC8406869
- DOI: 10.1093/cid/ciab630
Evaluating Vaccine Efficacy Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection
Abstract
Although interim results from several large, placebo-controlled, phase 3 trials demonstrated high vaccine efficacy (VE) against symptomatic coronavirus disease 2019 (COVID-19), it is unknown how effective the vaccines are in preventing people from becoming asymptomatically infected and potentially spreading the virus unwittingly. It is more difficult to evaluate VE against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection than against symptomatic COVID-19 because infection is not observed directly but rather is known to occur between 2 antibody or reverse-transcription polymerase chain reaction (RT-PCR) tests. Additional challenges arise as community transmission changes over time and as participants are vaccinated on different dates because of staggered enrollment of participants or crossover of placebo recipients to the vaccine arm before the end of the study. Here, we provide valid and efficient statistical methods for estimating potentially waning VE against SARS-CoV-2 infection with blood or nasal samples under time-varying community transmission, staggered enrollment, and blinded or unblinded crossover. We demonstrate the usefulness of the proposed methods through numerical studies that mimic the BNT162b2 phase 3 trial and the Prevent COVID U study. In addition, we assess how crossover and the frequency of diagnostic tests affect the precision of VE estimates.
Keywords: asymptomatic infection; seroconversion; symptomatic COVID-19; viral RNA; waning efficacy.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
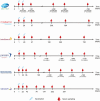
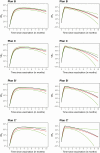
Update of
-
Evaluating Vaccine Efficacy Against SARS-CoV-2 Infection.medRxiv [Preprint]. 2021 Apr 17:2021.04.16.21255614. doi: 10.1101/2021.04.16.21255614. medRxiv. 2021. Update in: Clin Infect Dis. 2022 Feb 11;74(3):544-552. doi: 10.1093/cid/ciab630. PMID: 33880481 Free PMC article. Updated. Preprint.
References
-
- Janssen Biotech, Inc. FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. 2021. Available at: https://www.fda.gov/media/146217/download. Accessed 13 July 2021.
-
- Kalbfleisch JD, Prentice RL.. The statistical analysis of failure time data. 2nd ed.New York: John Wiley & Sons, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

