Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: Insights from the Caravaggio study
- PMID: 34260816
- PMCID: PMC9290511
- DOI: 10.1111/jth.15461
Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: Insights from the Caravaggio study
Abstract
Background: Clinical guidelines advise similar anticoagulant treatment for symptomatic and incidental cancer-associated venous thromboembolism (VTE). We investigated clinical features and outcomes of cancer patients with incidental or symptomatic VTE randomized in the Caravaggio study.
Objectives: We performed a predefined sub-analysis of the Caravaggio study in order to investigate the clinical features and outcomes of incidental and symptomatic VTE in patients with cancer. The relative efficacy and safety of apixaban and dalteparin in patients with incidental and symptomatic VTE was also assessed.
Methods: The Caravaggio study compared apixaban to dalteparin for the 6-month treatment of cancer-associated VTE. The primary efficacy and safety outcomes were recurrent VTE and major bleeding.
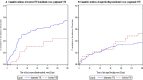
Results: Two hundred thirty patients (20%) had incidental and 925 (80%) symptomatic VTE. Pulmonary embolism with or without deep vein thrombosis as index event, colorectal cancer, Eastern Cooperative Oncology Group (ECOG) score of 0, and locally advanced or metastatic cancer were more frequent in patients with incidental VTE. Deep vein thrombosis as index event, hematological cancer, and ECOG score of 2 were more frequent in patients with symptomatic VTE. Ten patients (4.3%) with incidental and 68 (7.4%) with symptomatic VTE had recurrent VTE (hazard ratio [HR] 0.57, 95% confidence interval [CI] 0.29-1.10). Major bleeding occurred in 12 (5.2%) patients with incidental VTE and in 33 (3.6%) patients with symptomatic VTE (HR 1.43, 95% CI 0.74-2.77). When comparing apixaban to dalteparin in patients with symptomatic and incidental VTE, the HR for recurrence was 0.73 (95% CI 0.45-1.19) and 0.41 (95% CI 0.11-1.56), respectively, and the HR for major bleeding 0.93 (95% CI 0.47-1.83) and 0.96 (95% CI 0.31-2.96), respectively.
Conclusions: Compared to cancer patients with symptomatic VTE, those with incidental VTE have different clinical features at presentation, with a numerically lower incidence of recurrent VTE and a numerically higher incidence of major bleeding.
Trial registration: ClinicalTrials.gov NCT03045406.
Keywords: apixaban; cancer; incidental venous thromboembolism; symptomatic venous thromboembolism; venous thromboembolism.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Michela Giustozzi, Ana Belen Ruperez Blanco, Sebastian Szmit, Nicolas Falvo have nothing to disclose. Jean M Connors has received honoraria/consulting fees from Abbott, Bristol‐Myers Squibb, Pfizer, Takeda, and research funding to the institution from CSL Behring. Menno Huisman has received grants from ZonMw Dutch Healthcare Fund, Dutch Heart foundation, Boehringer‐Ingelheim, Pfizer‐BMS, Leo Pharma, Bayer Health Care. Rupert Bauersachs has received funding from Bayer, BMS, Boehringer Ingelheim, Daiichi‐Sankyo and Pfizer. Alexander T Cohen has received honoraria/consulting fees from a number of companies including AbbVie, Alexion, Bristol‐Myers Squibb, Pfizer, Bayer, Daiichi, and Sanofi, research funding from Boston Scientific, Bristol‐Myers Squibb, Pfizer, Bayer, and Daiichi. Francesco Dentali has received honoraria/consulting fee from Bayer, BMS, Boehringer Ingelheim, Daiichi‐Sankyo and Pfizer. Cecilia Becattini reports personal fees from Bristol Myers Squibb, Pfizer, Bayer Healthcare, and Daichi Sankyo outside the submitted work. Giancarlo Agnelli reports personal fees from Bristol Myers Squibb, Pfizer, Bayer Healthcare, and Daichi Sankyo outside the submitted work.
Figures
References
-
- Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population‐based cohort study. Thromb Haemost. 2017;117(1):57‐65. - PubMed
-
- Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Health. 2018;21(4):449‐455. - PubMed
-
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543‐603. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical