Resistance Correlations Influence Infection by Foreign Pathogens
- PMID: 34260867
- PMCID: PMC8283004
- DOI: 10.1086/715013
Resistance Correlations Influence Infection by Foreign Pathogens
Abstract
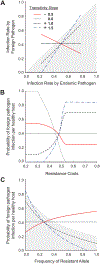
AbstractReciprocal selection promotes the specificity of host-pathogen associations and resistance polymorphisms in response to disease. However, plants and animals also vary in response to pathogen species not previously encountered in nature, with potential effects on new disease emergence. Using anther smut disease, we show that resistance (measured as infection rates) to foreign pathogens can be correlated with standing variation in resistance to an endemic pathogen. In Silene vulgaris, genetic variation in resistance to its endemic anther smut pathogen correlated positively with resistance variation to an anther smut pathogen from another host, but the relationship was negative between anther smut and a necrotrophic pathogen. We present models describing the genetic basis for assessing resistance relationships between endemic and foreign pathogens and for quantifying infection probabilities on foreign pathogen introduction. We show that even when the foreign pathogen has a lower average infection ability than the endemic pathogen, infection outcomes are determined by the sign and strength of the regression of the host's genetic variation in infection rates by a foreign pathogen on variation in infection rates by an endemic pathogen as well as by resistance allele frequencies. Given that preinvasion equilibria of resistance are determined by factors including resistance costs, we show that protection against foreign pathogens afforded by positively correlated resistances can be lessened or even result in elevated infection risk at the population level, depending on local dynamics. Therefore, a pathogen's emergence potential could be influenced not only by its average infection rate but also by resistance variation resulting from prior selection imposed by endemic diseases.
Keywords: Microbotryum; disease emergence; disease resistance; host shift.
Figures
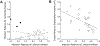
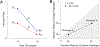
References
Literature Cited
-
- Abrams PA and Cortez MH. 2015. The many potential indirect interactions between predators that share competing prey. Ecological Monographs 85:625–641.
-
- Alexander HM 1989. An experimental field study of anther-smut disease of Silene alba caused by Ustilago violacea: genotypic variation and disease incidence. Evolution 43:835–847. - PubMed
-
- Alexander HM and Antonovics J. 1995. Spread of anther-smut disease (Ustilago violacea) and character correlations in a genetically variable experimental population of Silene alba. Journal of Ecology 83:783–794.
-
- Alexander HM, Antonovics J, and Kelly AW. 1993. Genotypic variation in plant disease resistance-physiological resistance in relation to field disease transmission. Journal of Ecology 81:325–333.
References Cited Only in the Online Enhancements
-
- Fellowes MDE, Kraaijeveld AR, and Godfray HCJ. 1999. Cross-resistance following artificial selection for increased defense against parasitoids in Drosophila melanogaster. Evolution 53:966–972. - PubMed
-
- Fokunang CN, Ikotun T, Dixon AG, and Akem CN. 2000. Field reaction of cassava genotypes to anthracnose, bacterial blight, cassava mosaic disease and their effects on yield. African Crop Science Journal 8:179–186.
-
- Intemann CD, Thye T, Niemann S, Browne EN, Chinbuah MA, Enimil A, Gyapong J, Osei I, Owusu-Dabo E, Helm S, and Rüsch-Gerdes S. 2009. Autophagy gene variant IRGM− 261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathogens 5:e1000577. - PMC - PubMed
-
- Liu JJ, Ekramoddoullah AK, Piggott N, and Zamani A. 2005. Molecular cloning of a pathogen/wound-inducible PR10 promoter from Pinus monticola and characterization in transgenic Arabidopsis plants. Planta 221:159–169. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

