Sarcoidosis-Related Cardiomyopathy: Current Knowledge, Challenges, and Future Perspectives State-of-the-Art Review
- PMID: 34260889
- PMCID: PMC8748280
- DOI: 10.1016/j.cardfail.2021.06.016
Sarcoidosis-Related Cardiomyopathy: Current Knowledge, Challenges, and Future Perspectives State-of-the-Art Review
Abstract
The prevalence of sarcoidosis-related cardiomyopathy is increasing. Sarcoidosis impacts cardiac function through granulomatous infiltration of the heart, resulting in conduction disease, arrhythmia, and/or heart failure. The diagnosis of cardiac sarcoidosis (CS) can be challenging and requires clinician awareness as well as differentiation from overlapping diagnostic phenotypes, such as other forms of myocarditis and arrhythmogenic cardiomyopathy. Clinical manifestations, extracardiac involvement, histopathology, and advanced cardiac imaging can all lend support to a diagnosis of CS. The mainstay of therapy for CS is immunosuppression; however, no prospective clinical trials exist to guide management. Patients may progress to developing advanced heart failure or ventricular arrhythmia, for which ventricular assist device therapies or heart transplantation may be considered. The existing knowledge gaps in CS call for an interdisciplinary approach to both patient care and future investigation to improve mechanistic understanding and therapeutic strategies.
Keywords: cardiac sarcoidosis; heart transplantation; inflammatory cardiomyopathy; interdisciplinary; myocarditis.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


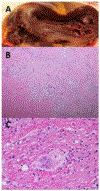

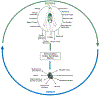
References
-
- Longcope WT. Sarcoidosis, or Besnier-Boeck-Schaumann Disease: The Frank Billings Lecture. JAMA. 1941;117(16):1321–7.
-
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361(9363):1111–8. - PubMed
-
- Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–32. - PubMed
-
- Yafasova A, Fosbol EL, Schou M, Gustafsson F, Rossing K, Bundgaard H, et al. Long-Term Adverse Cardiac Outcomes in Patients With Sarcoidosis. J Am Coll Cardiol. 2020;76(7):767–77. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

