Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment
- PMID: 34261093
- PMCID: PMC8711605
- DOI: 10.1097/QAD.0000000000003025
Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment
Abstract
Background: ATLAS (NCT02951052), a phase 3, multicenter, open-label study, demonstrated that switching to injectable cabotegravir (CAB) with rilpivirine (RPV) long-acting dosed every 4 weeks was noninferior at week (W) 48 to continuing three-drug daily oral current antiretroviral therapy (CAR). Results from the W 96 analysis are presented.
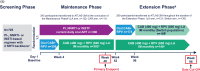
Methods and design: Participants completing W 52 of ATLAS were given the option to withdraw, transition to ATLAS-2M (NCT03299049), or enter an Extension Phase to continue long-acting therapy (Long-acting arm) or switch from CAR to long-acting therapy (Switch arm). Endpoints assessed at W 96 included proportion of participants with plasma HIV-1 RNA less than 50 copies/ml, incidence of confirmed virologic failure (CVF; two consecutive HIV-1 RNA ≥200 copies/ml), safety and tolerability, pharmacokinetics, and patient-reported outcomes.
Results: Most participants completing the Maintenance Phase transitioned to ATLAS-2M (88%, n = 502/572). Overall, 52 participants were included in the W 96 analysis of ATLAS; of these, 100% (n = 23/23) and 97% (n = 28/29) in the Long-acting and Switch arms had plasma HIV-1 RNA less than 50 copies/ml at W 96, respectively. One participant had plasma HIV-1 RNA 50 copies/ml or higher in the Switch arm (173 copies/ml). No participants met the CVF criterion during the Extension Phase. No new safety signals were identified. All Switch arm participants surveyed preferred long-acting therapy to their previous daily oral regimen (100%, n = 27/27).
Conclusion: In this subgroup of ATLAS, 98% (n = 51/52) of participants at the Extension Phase W 96 analysis maintained virologic suppression with long-acting therapy. Safety, efficacy, and participant preference results support the therapeutic potential of long-acting CAB+RPV treatment for virologically suppressed people living with HIV-1.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
S.S. reports grants from ViiV Healthcare, during the conduct of the study. T.L. received funding of studies from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, AbbVie, Gilead Sciences, Heidelberg ImmunoTherapeutics. L.V.Z., N.P., and E.M. have nothing to disclose. M.S. received honoraria as an advisor or lecturer, as well as funding of studies, from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, Novartis, and ViiV Healthcare. A.S. received honoraria as an advisor or lecturer, as well as funding of studies, from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, and ViiV Healthcare. H.C., R.V.S., S.V., and K.V. are employees and stockholders of Janssen, Pharmaceutical Companies of Johnson & Johnson. P.B., C.M.H., C.L.T., V.C., K.Y.S., and W.R.S. are employees of ViiV Healthcare and stockholders of GlaxoSmithKline. J.O.H., K.H., and S.L.F. are employees and stockholders of GlaxoSmithKline. D.A.M. was an employee of ViiV Healthcare and stockholder of GlaxoSmithKline during the conduct of the study and is now an employee of Brii Biosciences.
Figures
References
-
- U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2020. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/v.... [Accessed 25 February 2020]
-
- Kerrigan D, Mantsios A, Gorgolas M, Montes ML, Pulido F, Brinson C, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487. - PMC - PubMed
-
- De Los Rios P, Young B, Marcotullio S, Punekar Y, Koteff J, Ustianowski A, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the Positive Perspectives Study. Open Forum Infect Dis 2019; 6: (Suppl 2): S481.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous