Neuronal α-amylase is important for neuronal activity and glycogenolysis and reduces in presence of amyloid beta pathology
- PMID: 34261192
- PMCID: PMC8373367
- DOI: 10.1111/acel.13433
Neuronal α-amylase is important for neuronal activity and glycogenolysis and reduces in presence of amyloid beta pathology
Abstract
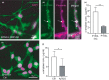
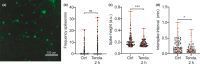
Recent studies indicate a crucial role for neuronal glycogen storage and degradation in memory formation. We have previously identified alpha-amylase (α-amylase), a glycogen degradation enzyme, located within synaptic-like structures in CA1 pyramidal neurons and shown that individuals with a high copy number variation of α-amylase perform better on the episodic memory test. We reported that neuronal α-amylase was absent in patients with Alzheimer's disease (AD) and that this loss corresponded to increased AD pathology. In the current study, we verified these findings in a larger patient cohort and determined a similar reduction in α-amylase immunoreactivity in the molecular layer of hippocampus in AD patients. Next, we demonstrated reduced α-amylase concentrations in oligomer amyloid beta 42 (Aβ42 ) stimulated SH-SY5Y cells and neurons derived from human-induced pluripotent stem cells (hiPSC) with PSEN1 mutation. Reduction of α-amylase production and activity, induced by siRNA and α-amylase inhibitor Tendamistat, respectively, was further shown to enhance glycogen load in SH-SY5Y cells. Both oligomer Aβ42 stimulated SH-SY5Y cells and hiPSC neurons with PSEN1 mutation showed, however, reduced load of glycogen. Finally, we demonstrate the presence of α-amylase within synapses of isolated primary neurons and show that inhibition of α-amylase activity with Tendamistat alters neuronal activity measured by calcium imaging. In view of these findings, we hypothesize that α-amylase has a glycogen degrading function within synapses, potentially important in memory formation. Hence, a loss of α-amylase, which can be induced by Aβ pathology, may in part underlie the disrupted memory formation seen in AD patients.
Keywords: Alzheimer's disease; alpha-amylases; amyloid beta-peptides; calcium imaging; glycogen; induced pluripotent stem cells; tendamistat.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Bass, B., Upson, S., Roy, K., Montgomery, E. L., Jalonen, T. O., & Murray, I. V. (2015). Glycogen and amyloid‐beta: Key players in the shift from neuronal hyperactivity to hypoactivity observed in Alzheimer's disease? Neural Regeneration Research, 10(7), 1023–1025. 10.4103/1673-5374.160059 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

