Neurophysiological biomarkers to optimize deep brain stimulation in movement disorders
- PMID: 34261338
- PMCID: PMC8977945
- DOI: 10.2217/nmt-2021-0002
Neurophysiological biomarkers to optimize deep brain stimulation in movement disorders
Abstract
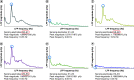
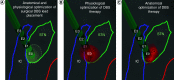
Intraoperative neurophysiological information could increase accuracy of surgical deep brain stimulation (DBS) lead placement. Subsequently, DBS therapy could be optimized by specifically targeting pathological activity. In Parkinson's disease, local field potentials (LFPs) excessively synchronized in the beta band (13-35 Hz) correlate with akinetic-rigid symptoms and their response to DBS therapy, particularly low beta band suppression (13-20 Hz) and high frequency gamma facilitation (35-250 Hz). In dystonia, LFPs abnormally synchronize in the theta/alpha (4-13 Hz), beta and gamma (60-90 Hz) bands. Phasic dystonic symptoms and their response to DBS correlate with changes in theta/alpha synchronization. In essential tremor, LFPs excessively synchronize in the theta/alpha and beta bands. Adaptive DBS systems will individualize pathological characteristics of neurophysiological signals to automatically deliver therapeutic DBS pulses of specific spatial and temporal parameters.
Keywords: Parkinson’s disease; deep brain stimulation; dystonia; essential tremor; local field potentials; microelectrode recordings; neuromodulation; neurophysiological biomarkers.
Conflict of interest statement
KJ Lizarraga and C Zimmerman have been reimbursed for travel to educational activities sponsored by Abbott, Boston Scientific and Medtronic. KJ Lizarraga has received an educational grant by Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Chen CC, Brücke C, Kempf F et al. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr. Biol. 16(22), R952–953 (2006). - PubMed
-
- Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov. Disord. 21(Suppl. 14), S259–S283 (2006). - PubMed
-
- Burchiel KJ, McCartney S, Lee A, Raslan AM. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J. Neurosurg. 119(2), 301–306 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
