Treatment outcomes among children younger than five years living with HIV in rural Zambia, 2008-2018: a cohort study
- PMID: 34261465
- PMCID: PMC8278691
- DOI: 10.1186/s12887-021-02793-y
Treatment outcomes among children younger than five years living with HIV in rural Zambia, 2008-2018: a cohort study
Abstract
Background: HIV testing and treatment guidelines for children in sub-Saharan Africa have evolved over time, such that children are now treated at younger ages. The objective of this study was to describe the treatment experience for immunologic, virologic, and growth outcomes among HIV-infected Zambian children younger than 5 years of age from 2008 to 2018.
Methods: Participants enrolled in a clinical cohort study in Macha, Zambia and initiating antiretroviral treatment before 5 years of age between 2008 and 2015 were included in the analysis and followed up to the end of 2018. Outcomes, including growth, CD4+ T-cell percentage, viral suppression, and mortality, were evaluated among all children using longitudinal and survival analyses. Comparisons by age at treatment initiation (< 1, 1 to < 2, and 2 to < 5 years) were also evaluated.
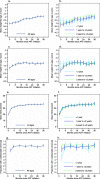
Results: Three hundred eighty-one children initiating treatment before 5 years of age between 2008 and 2015 were included in the analysis. Growth metrics and CD4+ T-cell percentage improved over time after treatment initiation. However, 20% of children remained underweight and 40% of children remained stunted after the first 36 months of treatment. 85% of children had a viral load < 400 copies/mL after 12 months of treatment. However, children < 1 year at treatment initiation were more likely to have a detectable viral load in the first 12 months of treatment and less likely to achieve viral suppression compared to older children. Mortality was highest in the first 12 months of treatment, among underweight children, and among children initiating treatment in 2008-2010 compared to 2011-2015.
Conclusions: Most children initiating antiretroviral treatment from 2008 to 2015 in rural Zambia responded well to treatment. However, many children remained underweight and stunted, and experienced high mortality rates during the first few months of treatment. This supports continued efforts to improve early infant diagnosis, nutritional support, and pediatric drug formulations.
Keywords: Antiretroviral therapy; HIV; Pediatrics; Sub-Saharan Africa.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization . WHO HIV update: global epidemic, progress in scale-up and policy uptake. Geneva: World Health Organization; 2020.
-
- World Health Organization. Scaling up antiretroviral therapy in resource-limited settings. Geneva: World Health Organization; 2002.
-
- Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, Josipovic D, Liberty A, Lazarus E, Innes S, et al. Early time-limited antiretroviral therapy versus deferred therapy in south African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–1563. doi: 10.1016/S0140-6736(13)61409-9. - DOI - PMC - PubMed
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2. Gevena: World Health Organization; 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

