Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly
- PMID: 34261503
- PMCID: PMC8278651
- DOI: 10.1186/s13059-021-02427-7
Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly
Abstract
Background: Akkermansia muciniphila is a human gut microbe with a key role in the physiology of the intestinal mucus layer and reported associations with decreased body mass and increased gut barrier function and health. Despite its biomedical relevance, the genomic diversity of A. muciniphila remains understudied and that of closely related species, except for A. glycaniphila, unexplored.
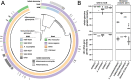
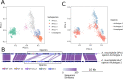
Results: We present a large-scale population genomics analysis of the Akkermansia genus using 188 isolate genomes and 2226 genomes assembled from 18,600 metagenomes from humans and other animals. While we do not detect A. glycaniphila, the Akkermansia strains in the human gut can be grouped into five distinct candidate species, including A. muciniphila, that show remarkable whole-genome divergence despite surprisingly similar 16S rRNA gene sequences. These candidate species are likely human-specific, as they are detected in mice and non-human primates almost exclusively when kept in captivity. In humans, Akkermansia candidate species display ecological co-exclusion, diversified functional capabilities, and distinct patterns of associations with host body mass. Analysis of CRISPR-Cas loci reveals new variants and spacers targeting newly discovered putative bacteriophages. Remarkably, we observe an increased relative abundance of Akkermansia when cognate predicted bacteriophages are present, suggesting ecological interactions. A. muciniphila further exhibits subspecies-level genetic stratification with associated functional differences such as a putative exo/lipopolysaccharide operon.
Conclusions: We uncover a large phylogenetic and functional diversity of the Akkermansia genus in humans. This variability should be considered in the ongoing experimental and metagenomic efforts to characterize the health-associated properties of A. muciniphila and related bacteria.
© 2021. The Author(s).
Conflict of interest statement
WMdV is co-founder and holds stock in A-mansia Biotech Belgium. All the other authors declare that they have no competing interests.
Figures



References
-
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium [Internet]. Int J Syst Evol Microbiol. 2004:1469–76 Available from: 10.1099/ijs.0.02873-0. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

