In vitro affinity maturation of broader and more-potent variants of the HIV-1-neutralizing antibody CAP256-VRC26.25
- PMID: 34261793
- PMCID: PMC8307357
- DOI: 10.1073/pnas.2106203118
In vitro affinity maturation of broader and more-potent variants of the HIV-1-neutralizing antibody CAP256-VRC26.25
Abstract
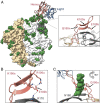
Three variable 2 (V2) loops of HIV-1 envelope glycoprotein (Env) trimer converge at the Env apex to form the epitope of an important classes of HIV-1 broadly neutralizing antibodies (bNAbs). These V2-glycan/apex antibodies are exceptionally potent but less broad (∼60 to 75%) than many other bNAbs. Their CDRH3 regions are typically long, acidic, and tyrosine sulfated. Tyrosine sulfation complicates efforts to improve these antibodies through techniques such as phage or yeast display. To improve the breadth of CAP256-VRC26.25 (VRC26.25), a very potent apex antibody, we adapted and extended a B cell display approach. Specifically, we used CRISPR/Cas12a to introduce VRC26.25 heavy- and light-chain genes into their respective loci in a B cell line, ensuring that each cell expresses a single VRC26.25 variant. We then diversified these loci through activation-induced cytidine deaminase-mediated hypermutation and homology-directed repair using randomized CDRH3 sequences as templates. Iterative sorting with soluble Env trimers and further randomization selected VRC26.25 variants with successively improving affinities. Three mutations in the CDRH3 region largely accounted for this improved affinity, and VRC26.25 modified with these mutations exhibited greater breadth and potency than the original antibody. Our data describe a broader and more-potent form of VRC26.25 as well as an approach useful for improving the breadth and potency of antibodies with functionally important posttranslational modifications.
Keywords: B cell display; CAP256-VRC26.25; V2-glycan bNAbs; affinity maturation; tyrosine sulfation.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

