Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade
- PMID: 34261797
- PMCID: PMC8449934
- DOI: 10.1126/scitranslmed.abb3631
Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade
Abstract
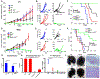
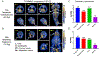
Molecular and cellular effects of radiotherapy on tumor microenvironment (TME) can help prime and propagate antitumor immunity. We hypothesized that delivering radiation to all tumor sites could augment response to immunotherapies. We tested an approach to enhance response to immune checkpoint inhibitors (ICIs) by using targeted radionuclide therapy (TRT) to deliver radiation semiselectively to tumors. NM600, an alkylphosphocholine analog that preferentially accumulates in most tumor types, chelates a radioisotope and semiselectively delivers it to the TME for therapeutic or diagnostic applications. Using serial 86Y-NM600 positron emission tomography (PET) imaging, we estimated the dosimetry of 90Y-NM600 in immunologically cold syngeneic murine models that do not respond to ICIs alone. We observed strong therapeutic efficacy and reported optimal dose (2.5 to 5 gray) and sequence for 90Y-NM600 in combination with ICIs. After combined treatment, 45 to 66% of mice exhibited complete response and tumor-specific T cell memory, compared to 0% with 90Y-NM600 or ICI alone. This required expression of STING in tumor cells. Combined TRT and ICI activated production of proinflammatory cytokines in the TME, promoted tumor infiltration by and clonal expansion of CD8+ T cells, and reduced metastases. In mice bearing multiple tumors, combining TRT with moderate-dose (12 gray) external beam radiotherapy (EBRT) targeting a single tumor augmented response to ICIs compared to combination of ICIs with either TRT or EBRT alone. The safety of TRT was confirmed in a companion canine study. Low-dose TRT represents a translatable approach to promote response to ICIs for many tumor types, regardless of location.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures






References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD, Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma, N Engl J Med 373, 23–34 (2015). - PMC - PubMed
-
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS, Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma, N Engl J Med 372, 2006–2017 (2015). - PMC - PubMed
-
- Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D’Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba II, Baker LH, Maki RG, Reinke D, Patel S, Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial, The Lancet Oncology 18, 1493–1501 (2017). - PMC - PubMed
-
- Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M, Polson K, Loucks M, Severgnini M, Patel T, Cunningham A, Rodig SJ, Hodi FS, Morgan JA, Merriam P, Wagner AJ, Shapiro GI, George S, Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study: Nivolumab for Uterine Leiomyosarcoma, Cancer 123, 3285–3290 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 CA250263/CA/NCI NIH HHS/United States
- K08 CA241319/CA/NCI NIH HHS/United States
- P50 DE026787/DE/NIDCR NIH HHS/United States
- TL1 TR002375/TR/NCATS NIH HHS/United States
- R35 CA197078/CA/NCI NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- U01 CA233102/CA/NCI NIH HHS/United States
- U54 CA232568/CA/NCI NIH HHS/United States
- U54 HD090256/HD/NICHD NIH HHS/United States
- DP5 OD024576/OD/NIH HHS/United States
- P01 CA250972/CA/NCI NIH HHS/United States
- T32 CA009206/CA/NCI NIH HHS/United States
- T32 GM008692/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

